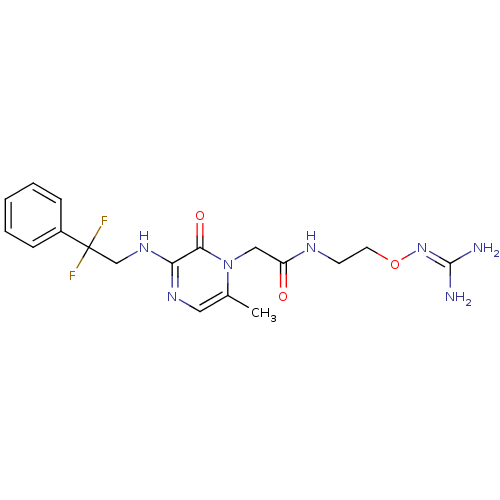
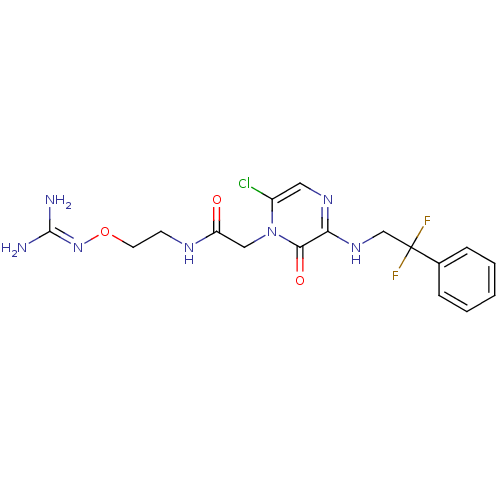
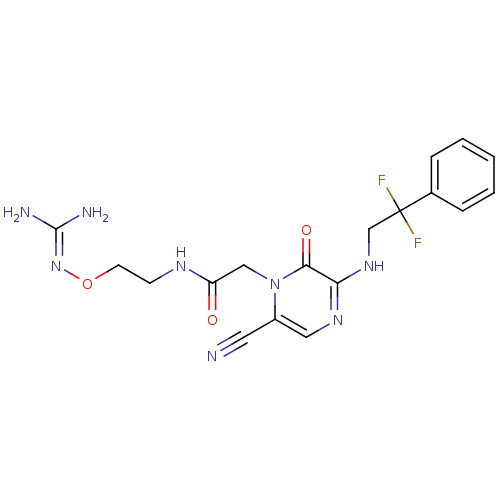
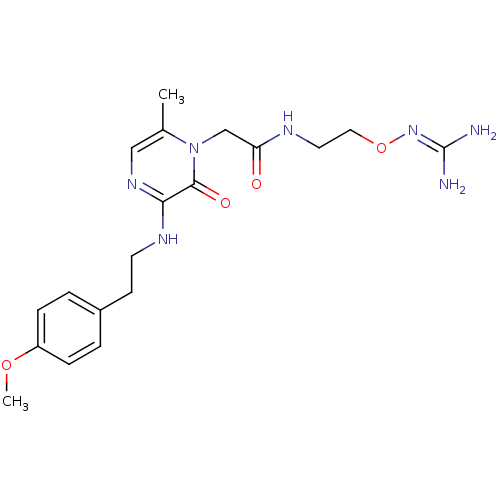
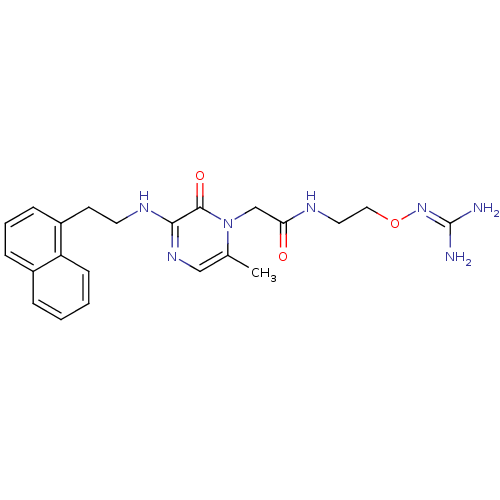
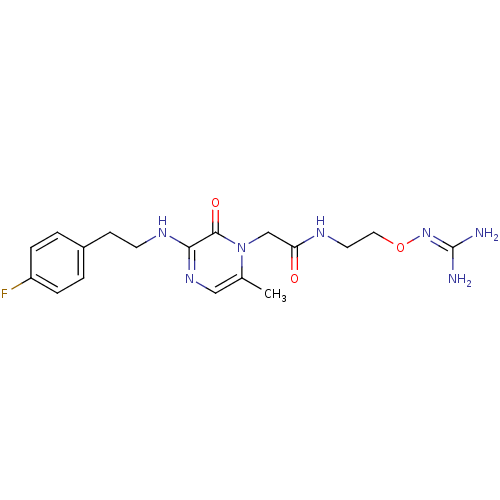
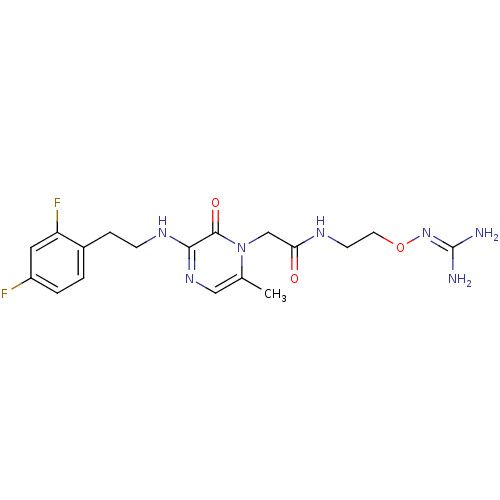
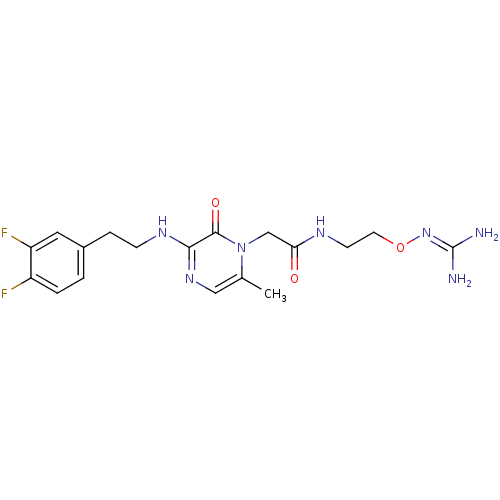
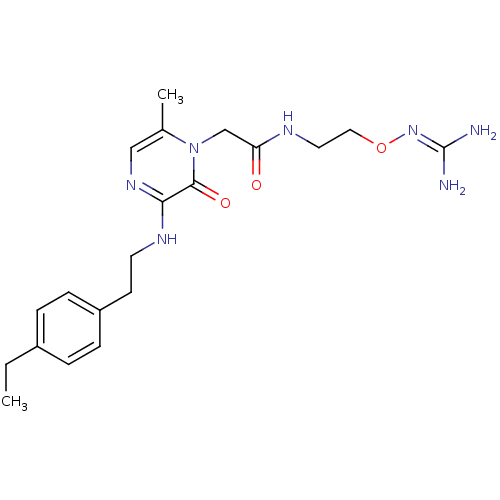
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
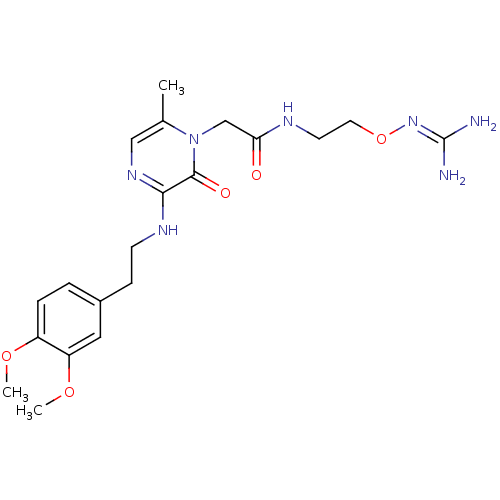
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 6.80nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 37nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 44nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 47nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 47nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 66nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 120nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetTissue-type plasminogen activator(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2.40E+3nMAssay Description:Inhibition of tissue-type plasminogen activatorMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 3.30E+3nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 6.50E+3nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of human leukocyte elastaseMore data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of plasminMore data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of plasminMore data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of human leukocyte elastaseMore data for this Ligand-Target Pair
TargetCathepsin G(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of cathepsin GMore data for this Ligand-Target Pair
TargetCathepsin G(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of cathepsin GMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of urokinaseMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of urokinaseMore data for this Ligand-Target Pair
TargetCoagulation factor VII(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of factor 7aMore data for this Ligand-Target Pair
TargetCoagulation factor VII(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of factor 7aMore data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of plasminMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of chymaseMore data for this Ligand-Target Pair
TargetNeutrophil elastase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of human leukocyte elastaseMore data for this Ligand-Target Pair
TargetCathepsin G(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of cathepsin GMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of urokinaseMore data for this Ligand-Target Pair
TargetCoagulation factor VII(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of factor 7aMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of chymaseMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: >2.60E+4nMAssay Description:Inhibition of chymaseMore data for this Ligand-Target Pair