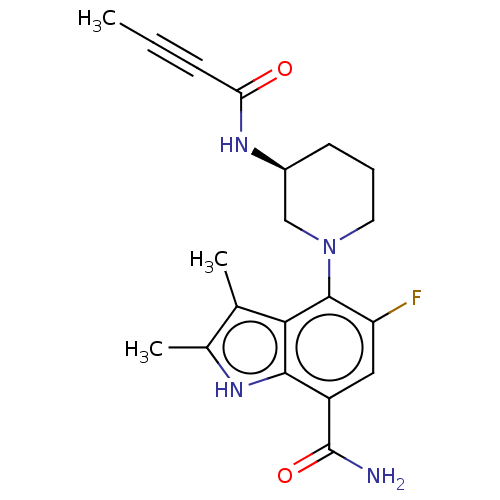
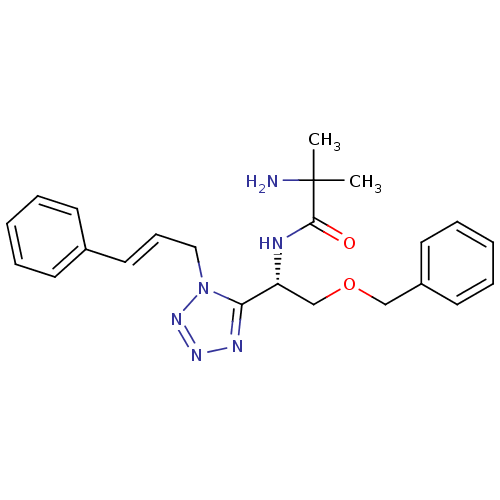
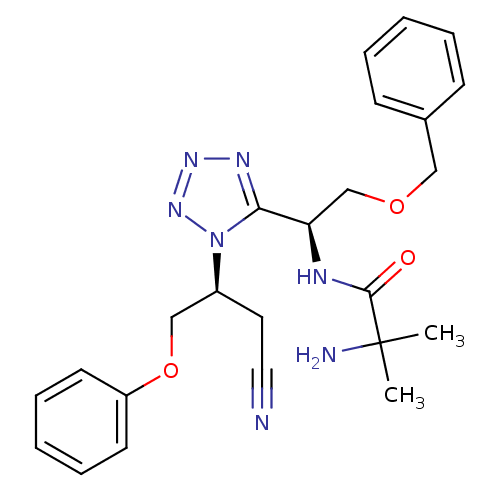
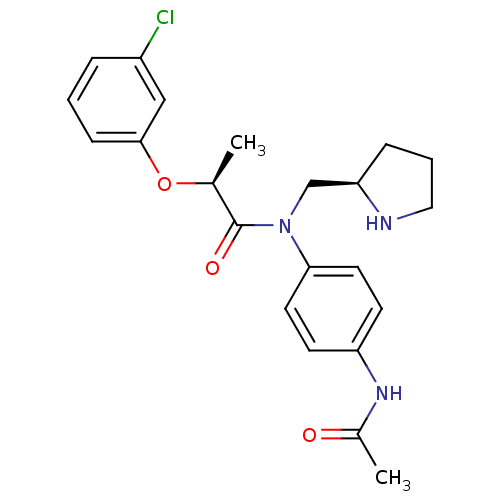
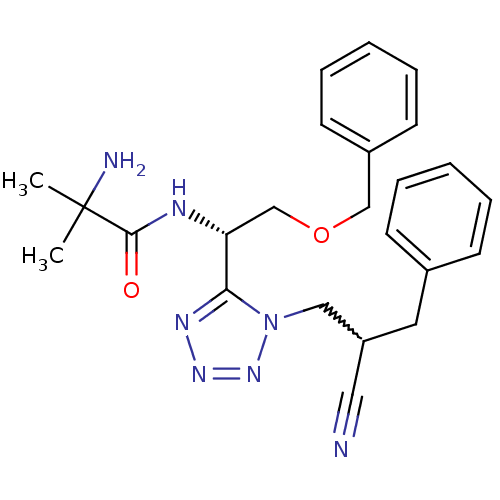
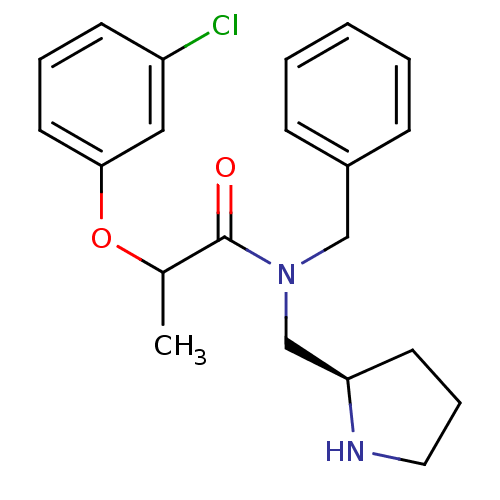
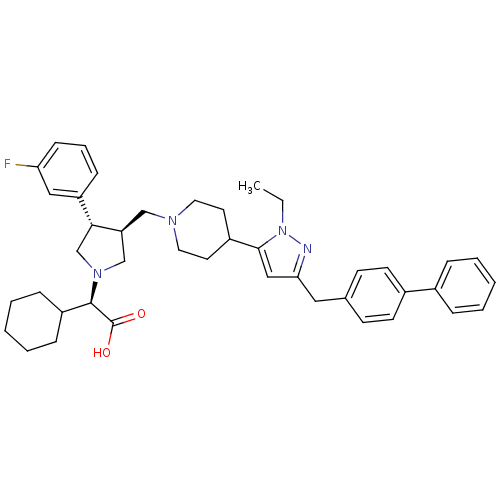
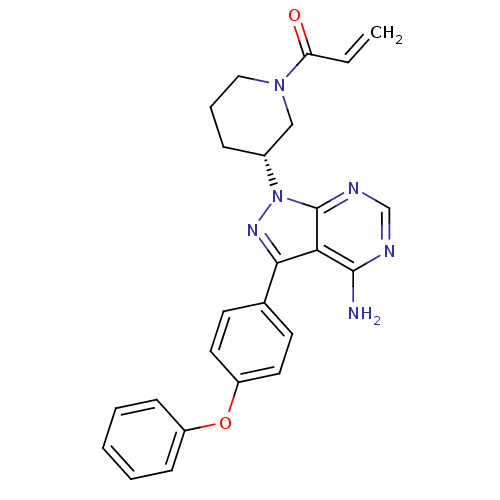
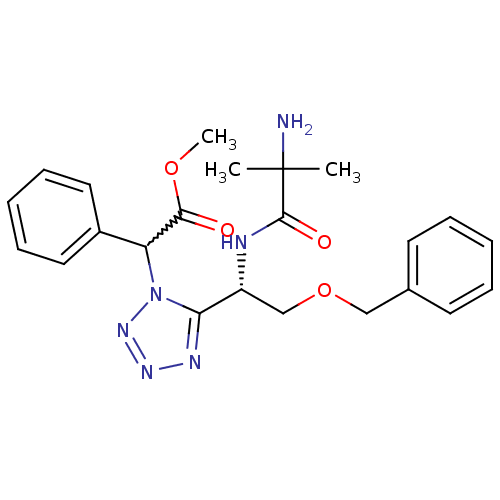
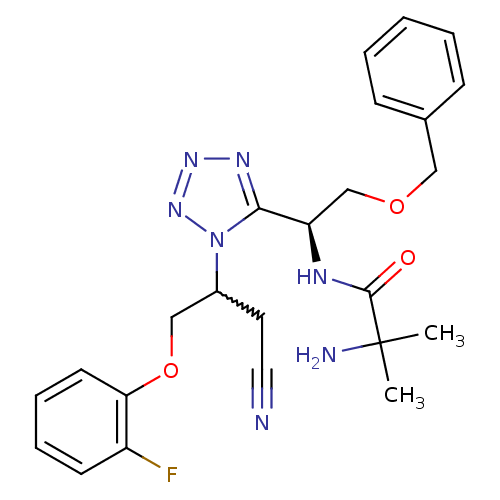
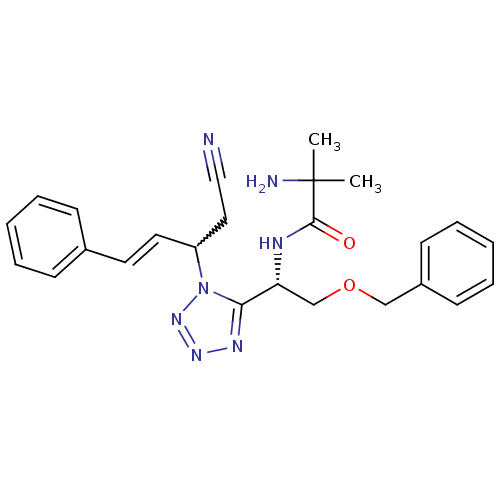
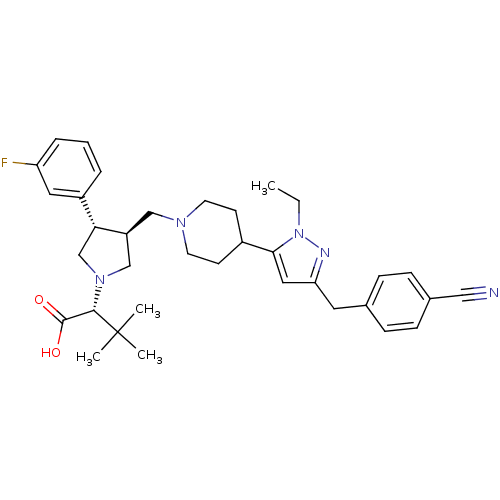
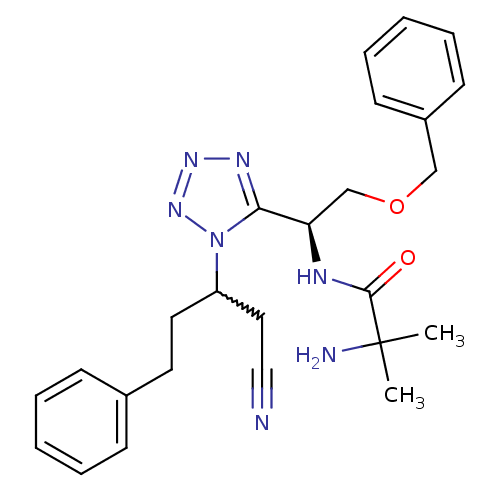
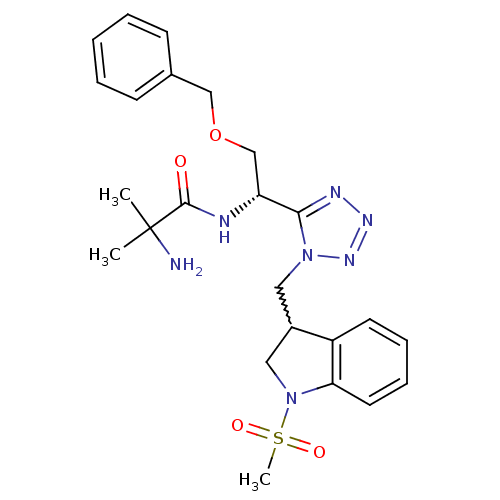
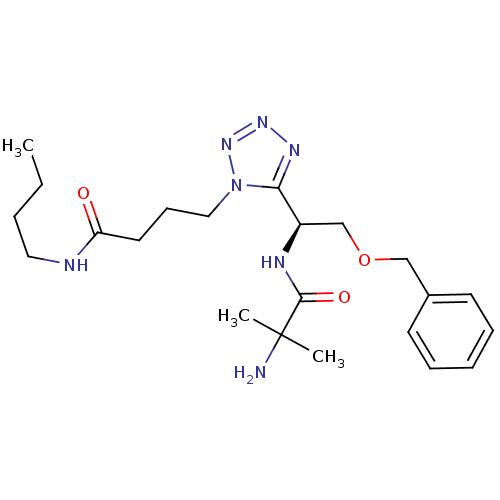
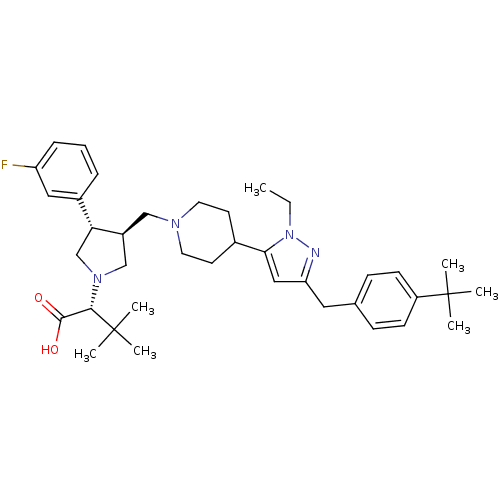
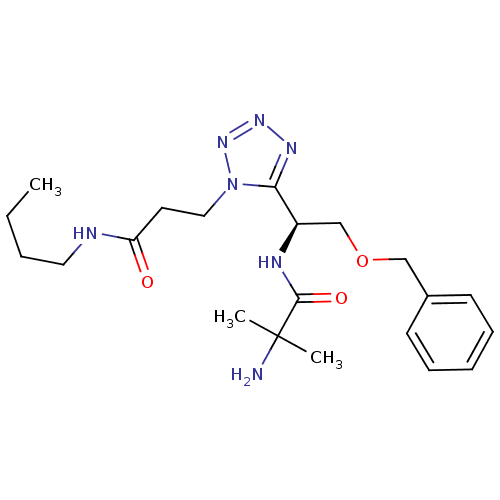
TargetMotilin receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.700nMAssay Description:In vitro binding affinity towards human motilin receptorMore data for this Ligand-Target Pair
TargetMotilin receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:In vitro binding affinity towards human motilin receptorMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Inhibitory activity of the compound against specific binding of [125I]-MIP-1 alpha to human CCR5 receptorMore data for this Ligand-Target Pair
TargetMotilin receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:In vitro binding affinity towards human motilin receptorMore data for this Ligand-Target Pair
TargetMotilin receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 2.70nMAssay Description:In vitro binding affinity towards human motilin receptorMore data for this Ligand-Target Pair
TargetMotilin receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.10nMAssay Description:In vitro binding affinity towards human motilin receptorMore data for this Ligand-Target Pair
TargetMotilin receptor(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.40nMAssay Description:In vitro binding affinity towards human motilin receptorMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 5.70nMAssay Description:Agonist activity at human GHS receptor expressed in H4 glioma cellsMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 7.5nMAssay Description:Agonist activity at human GHS receptor expressed in H4 glioma cellsMore data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Inhibition constant against HIV protease was determinedMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 30nMAssay Description:Binding affinity to GHSR (unknown origin)More data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 30nMAssay Description:Binding affinity to GHSR (unknown origin)More data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Agonist activity at human GHS receptor expressed in H4 glioma cellsMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 90nMAssay Description:Binding affinity to GHSR (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:In vitro inhibition of HIV-1 protease.More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 116nMAssay Description:Inhibition of His-tagged BTK (unknown origin) after 1.5 hrs by HTRF analysisMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 190nMAssay Description:Displacement of [125I]Ghrelin from human GHSR1a after 1 hrMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 197nMAssay Description:Agonist activity at human GHS receptor expressed in H4 glioma cellsMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 200nMAssay Description:Displacement of [125I]Ghrelin from human GHSR1a after 1 hrMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 200nMAssay Description:Agonist activity at human GHS receptor expressed in H4 glioma cellsMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 240nMAssay Description:Agonist activity at human GHS receptor expressed in H4 glioma cellsMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 250nMAssay Description:Binding affinity to GHSR (unknown origin)More data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 260nMAssay Description:Binding affinity to GHSR (unknown origin)More data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 298nMAssay Description:Agonist activity at human GHS receptor expressed in H4 glioma cellsMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 300nMAssay Description:Binding affinity to GHSR (unknown origin)More data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 320nMAssay Description:Displacement of [125I]Ghrelin from human GHSR1a after 1 hrMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetTyrosine-protein kinase BTK(Homo sapiens (Human))
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Bristol-Myers Squibb Research And Development
Curated by ChEMBL
Affinity DataKi: 425nMAssay Description:Inhibition of His-tagged BTK (unknown origin) after 1.5 hrs by HTRF analysisMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 470nMAssay Description:Displacement of [125I]Ghrelin from human GHSR1a after 1 hrMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 503nMAssay Description:Agonist activity at human GHS receptor expressed in H4 glioma cellsMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 611nMAssay Description:Agonist activity at human GHS receptor expressed in H4 glioma cellsMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 696nMAssay Description:Agonist activity at human GHS receptor expressed in H4 glioma cellsMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 696nMAssay Description:Agonist activity at human GHS receptor expressed in H4 glioma cellsMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 737nMAssay Description:Agonist activity at human GHS receptor expressed in H4 glioma cellsMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 737nMAssay Description:Agonist activity at human GHS receptor expressed in H4 glioma cellsMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 780nMAssay Description:Displacement of [125I]Ghrelin from human GHSR1a after 1 hrMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 820nMAssay Description:Displacement of [125I]Ghrelin from human GHSR1a after 1 hrMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 970nMAssay Description:Displacement of [125I]Ghrelin from human GHSR1a after 1 hrMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 1.00E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.07E+3nMAssay Description:Displacement of [125I]Ghrelin from human GHSR1a after 1 hrMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.27E+3nMAssay Description:Displacement of [125I]Ghrelin from human GHSR1a after 1 hrMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.37E+3nMAssay Description:Displacement of [125I]Ghrelin from human GHSR1a after 1 hrMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.38E+3nMAssay Description:Agonist activity at human GHS receptor expressed in H4 glioma cellsMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.38E+3nMAssay Description:Agonist activity at human GHS receptor expressed in H4 glioma cellsMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.59E+3nMAssay Description:Displacement of [125I]Ghrelin from human GHSR1a after 1 hrMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.72E+3nMAssay Description:Displacement of [125I]Ghrelin from human GHSR1a after 1 hrMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.78E+3nMAssay Description:Displacement of [125I]Ghrelin from human GHSR1a after 1 hrMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.90E+3nMAssay Description:Displacement of [125I]Ghrelin from human GHSR1a after 1 hrMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.10E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 2.19E+3nMAssay Description:Displacement of [125I]Ghrelin from human GHSR1a after 1 hrMore data for this Ligand-Target Pair

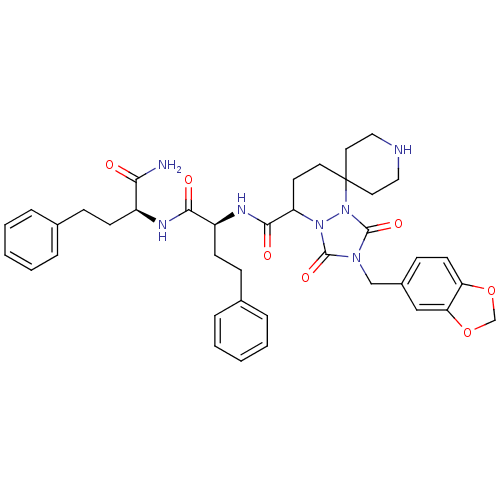

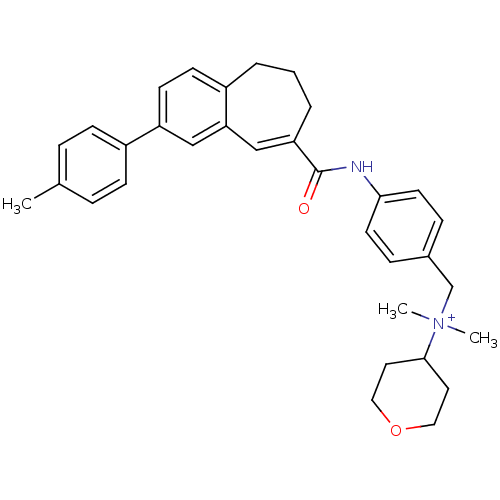
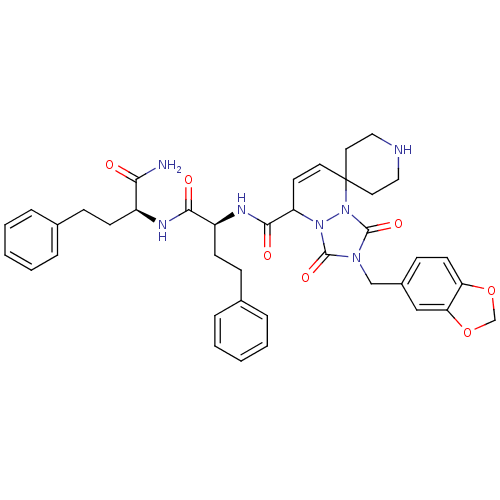

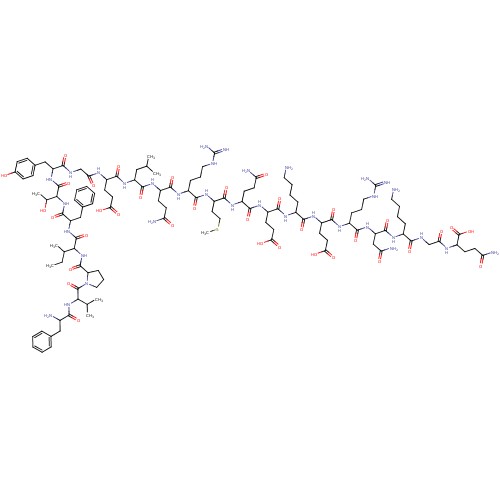
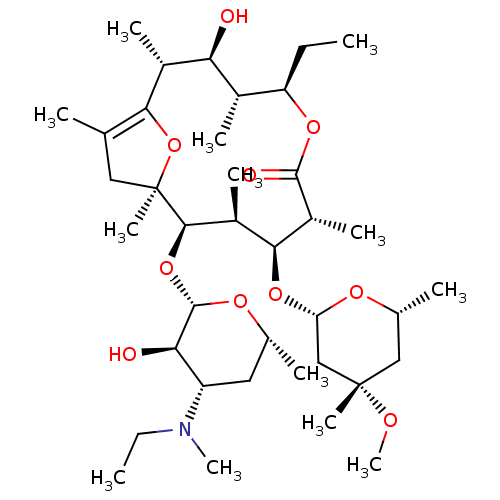





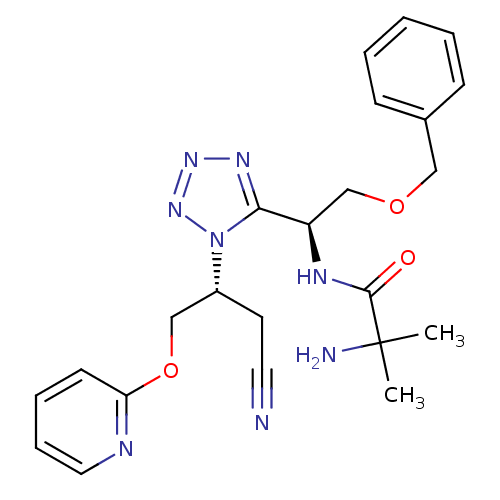
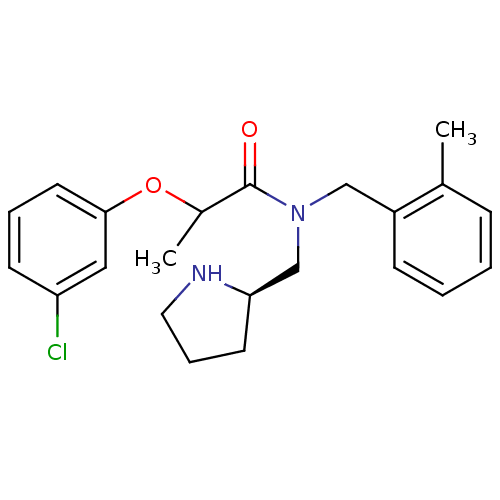
 3D Structure (crystal)
3D Structure (crystal)