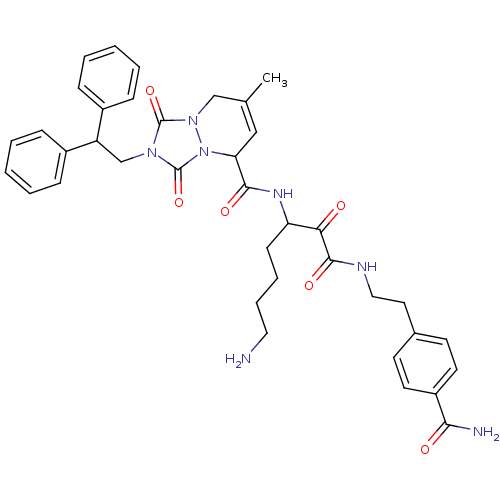
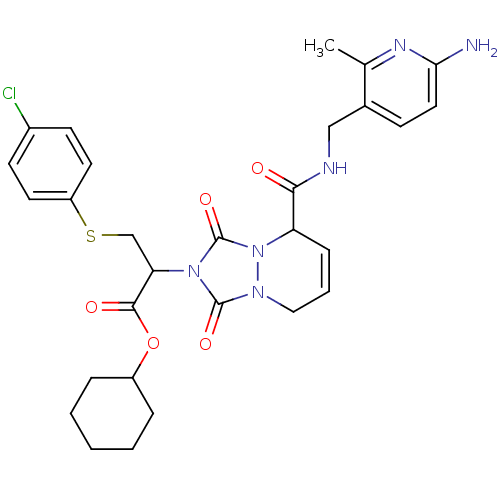
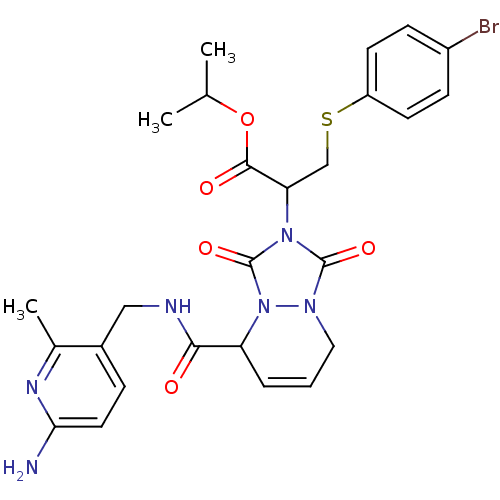
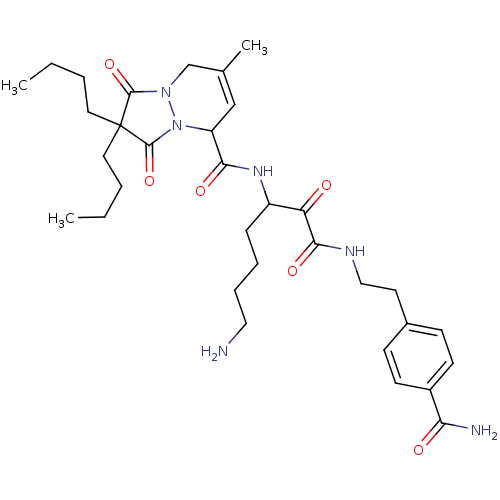
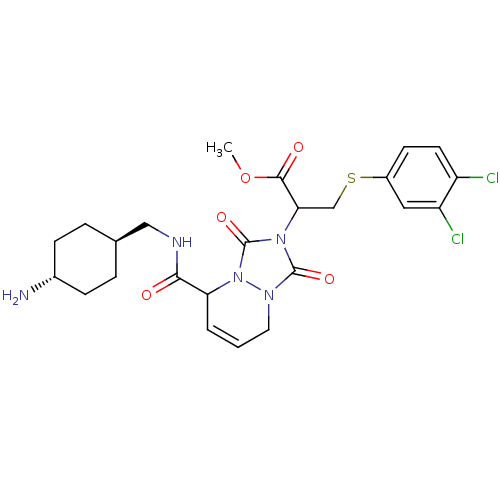
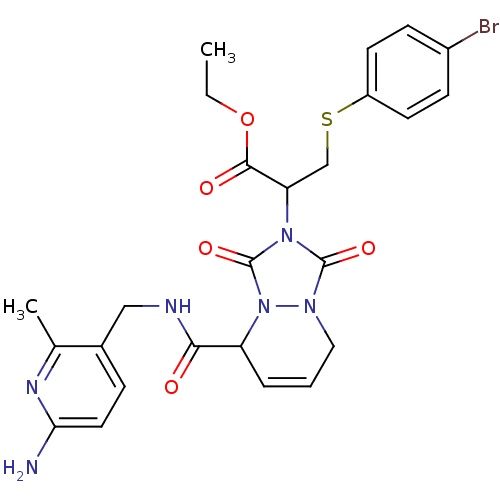
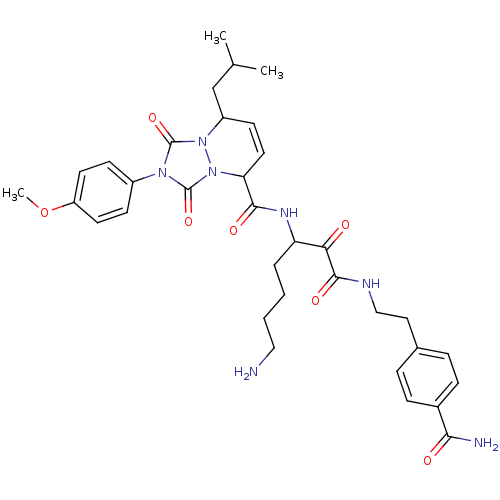
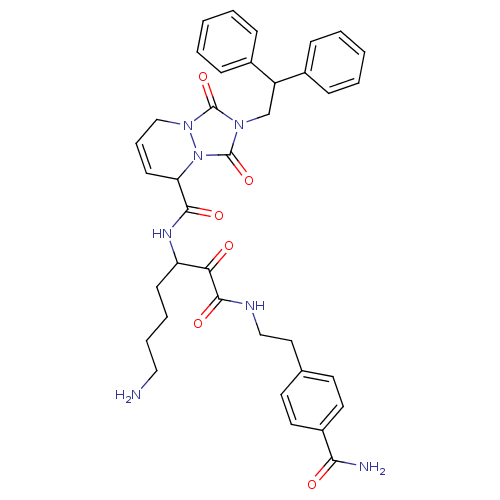
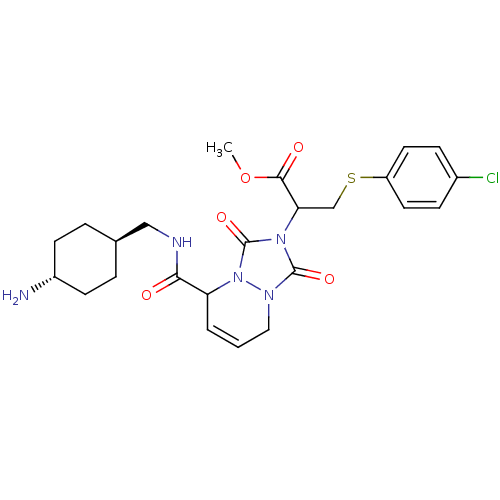
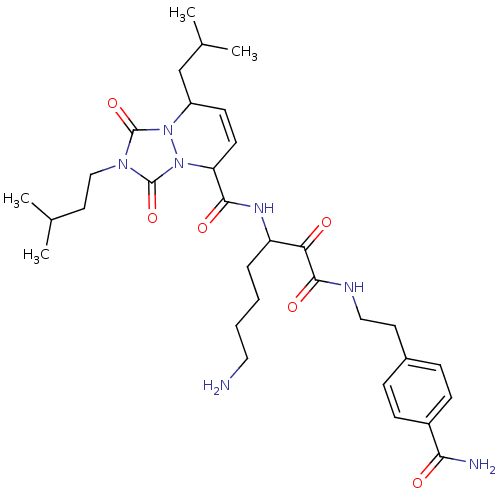
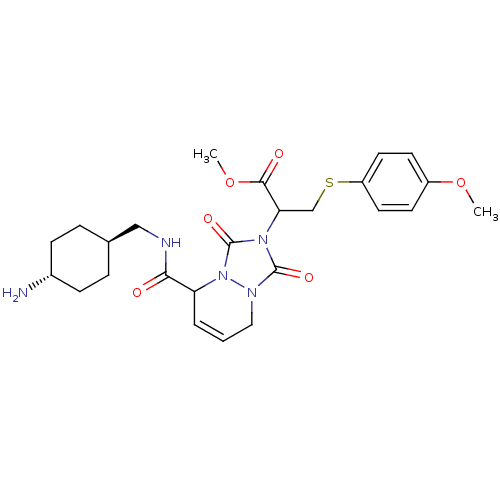
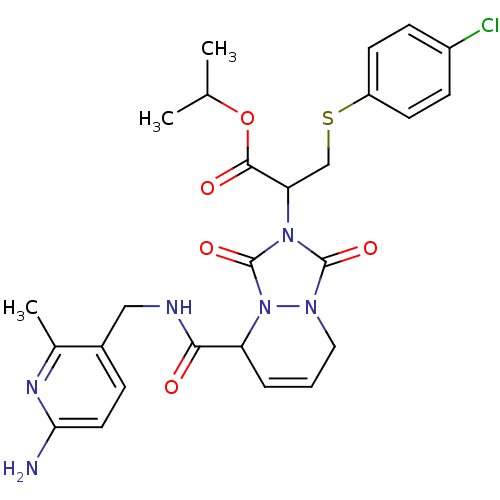
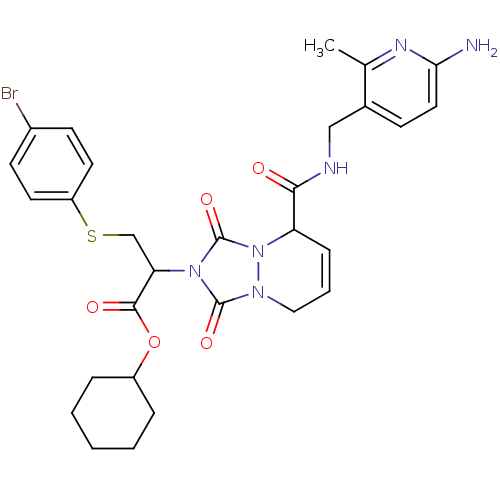
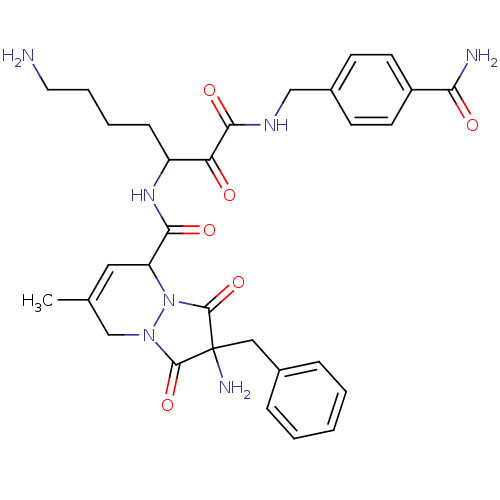
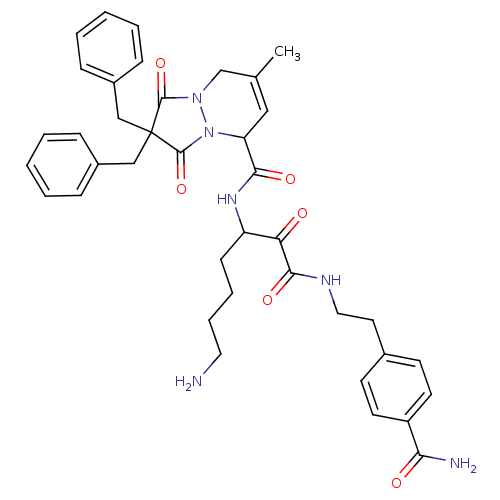
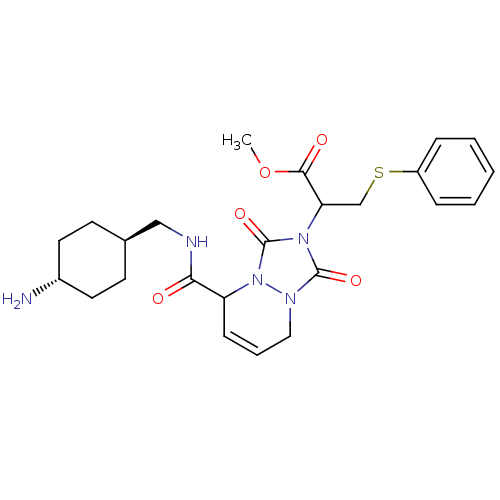
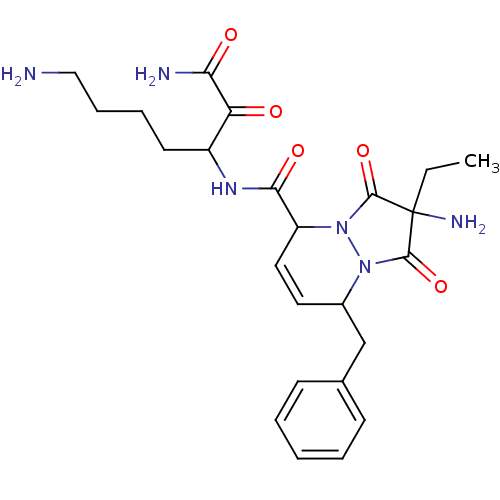
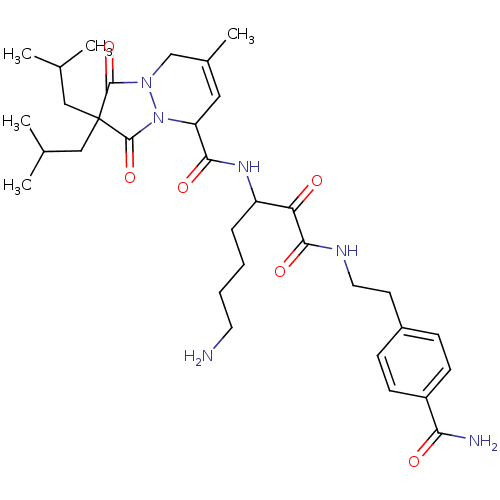
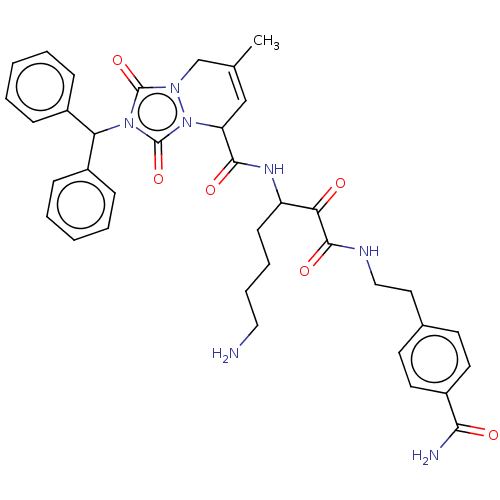
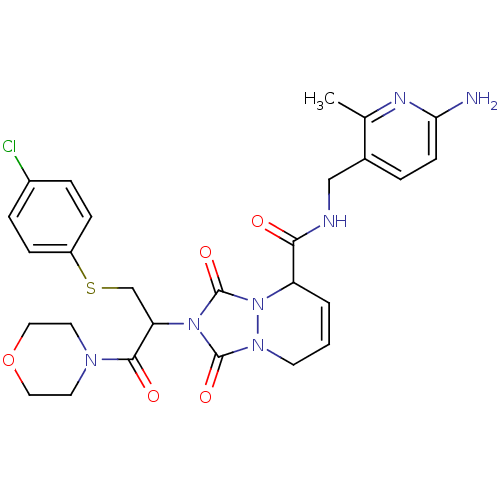
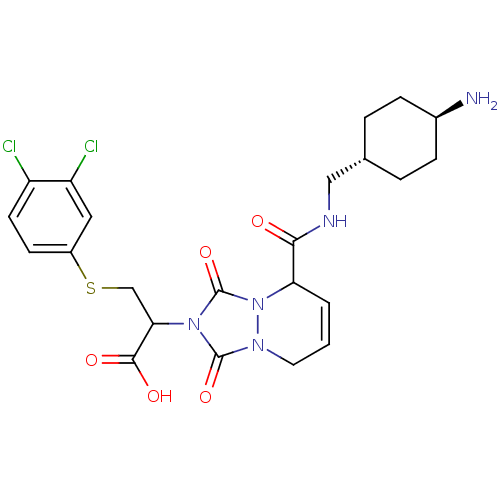
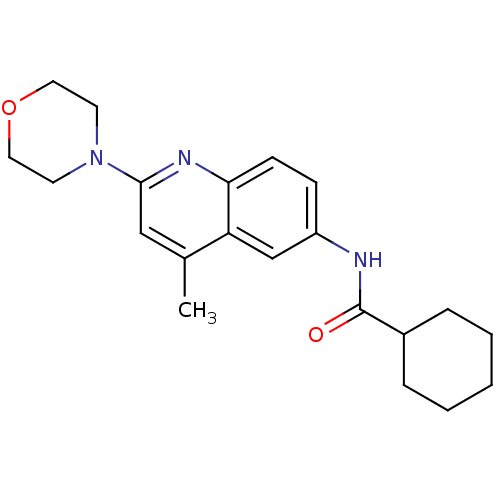
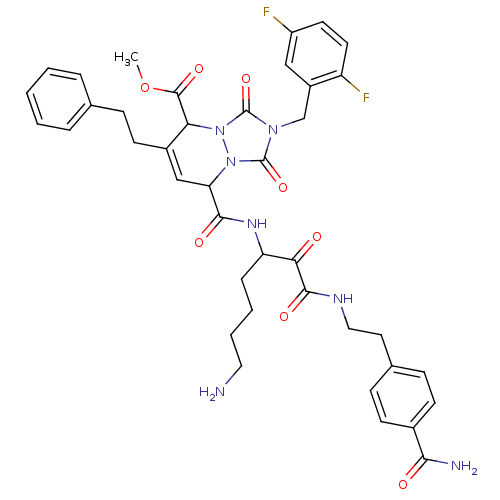
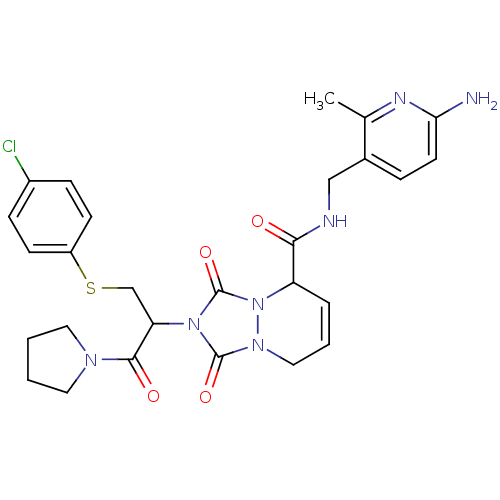
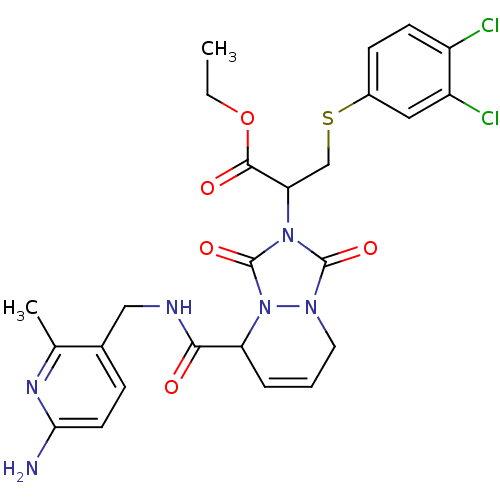
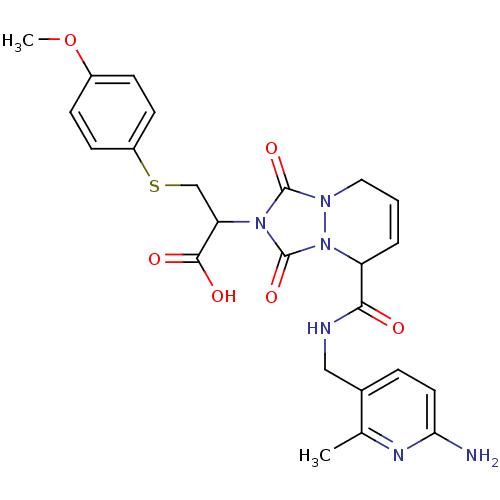
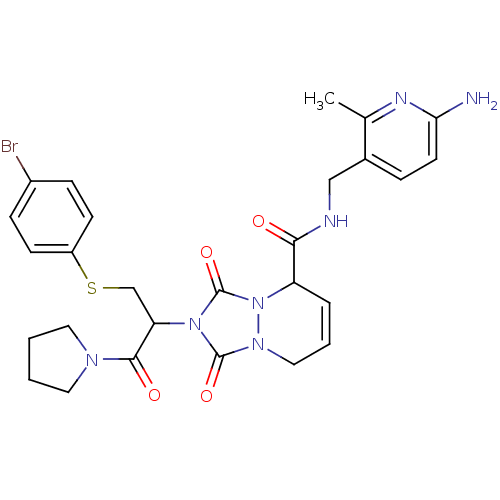
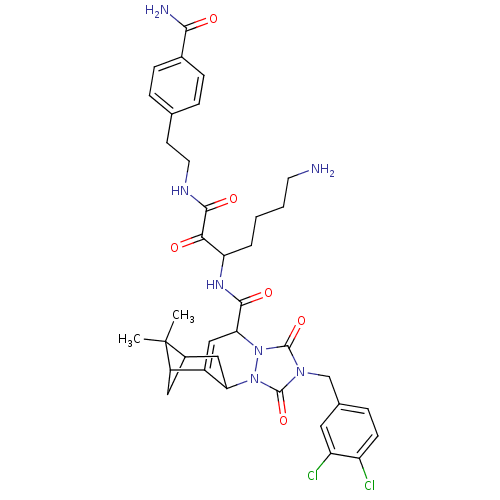
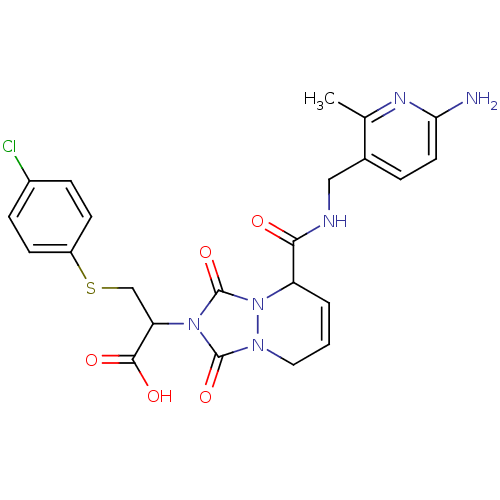
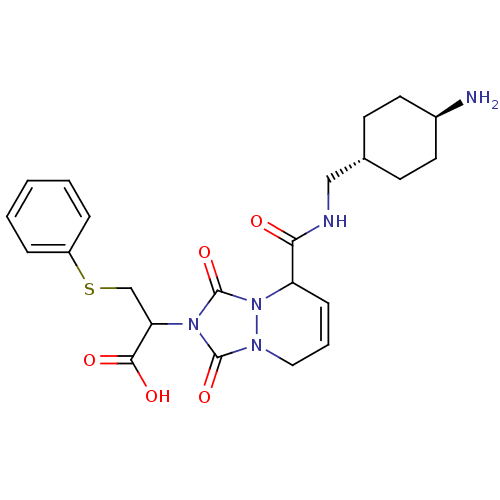
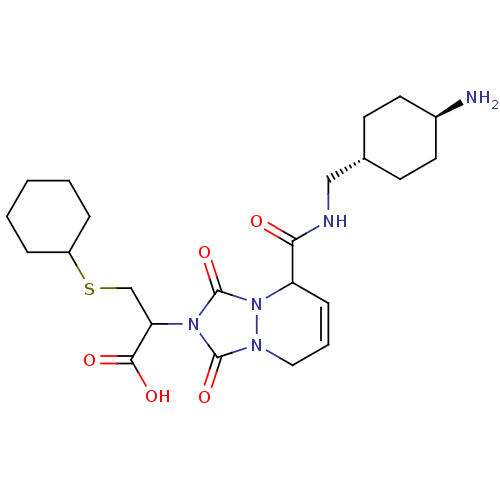
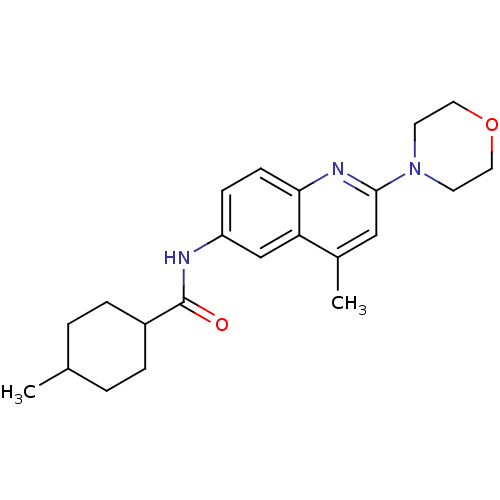
Affinity DataKi: 0.0230nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.0350nMAssay Description:Compound was evaluated for its binding affinity to the thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.0570nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.0870nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nMAssay Description:Compound was evaluated for its binding affinity to the thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.240nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.270nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.280nMAssay Description:Compound was evaluated for its binding affinity to the trypsin enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Compound was evaluated for its binding affinity to the thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.320nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 0.430nMAssay Description:Compound was evaluated for its binding affinity to the trypsin enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 0.75nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Compound was evaluated for its binding affinity to the thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 1.90nMAssay Description:Compound was evaluated for its binding affinity to the thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 2.90nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Compound was evaluated for its binding affinity to the thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 4.70nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 5.10nMAssay Description:Compound was evaluated for its binding affinity to the thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 5.70nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 5.90nMAssay Description:Compound was evaluated for its binding affinity to the tryptaseMore data for this Ligand-Target Pair
Affinity DataKi: 7.70nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Compound was evaluated for its binding affinity to the thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 21nM ΔG°: -43.2kJ/mole IC50: 31nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 21nMAssay Description:Compound was evaluated for its inhibitory activity against KallikreinMore data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 28nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 29nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 31nMAssay Description:Compound was evaluated for its inhibitory activity against KallikreinMore data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 36nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 52nM ΔG°: -41.0kJ/mole IC50: 103nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 53nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 53nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 55nM ΔG°: -40.9kJ/mole IC50: 133nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 56nM ΔG°: -40.8kJ/mole IC50: 63nMpH: 5.9 T: 2°CAssay Description:Enzyme activity was determined as the production of fluorescent resorufin from the substrate. The fluorescence was measured (excitation 570 nm, emiss...More data for this Ligand-Target Pair
Affinity DataKi: 78nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair
Affinity DataKi: 82nMAssay Description:Inhibitory activity of the compound against human thrombinMore data for this Ligand-Target Pair