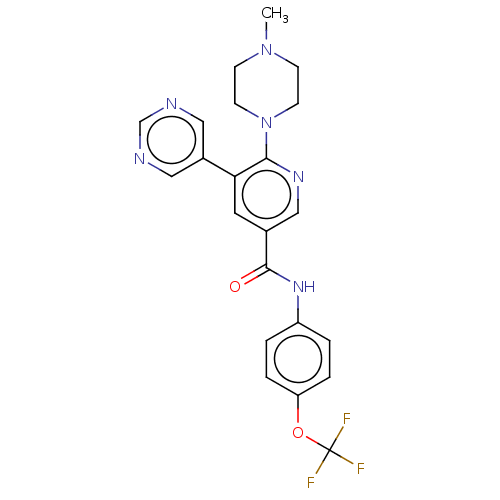
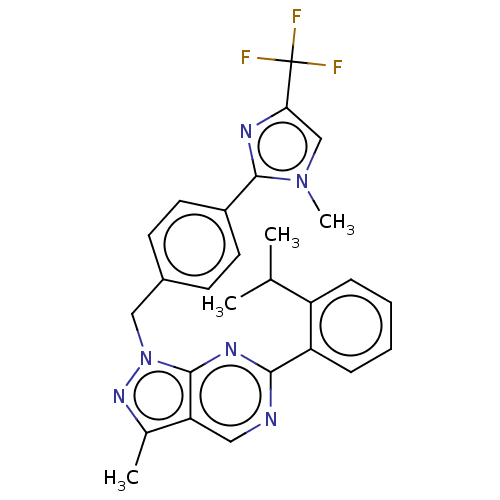
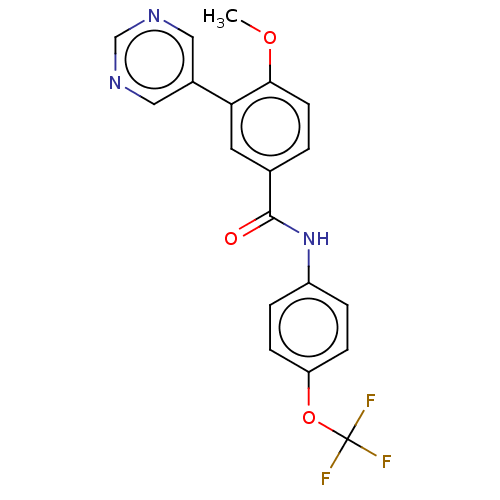
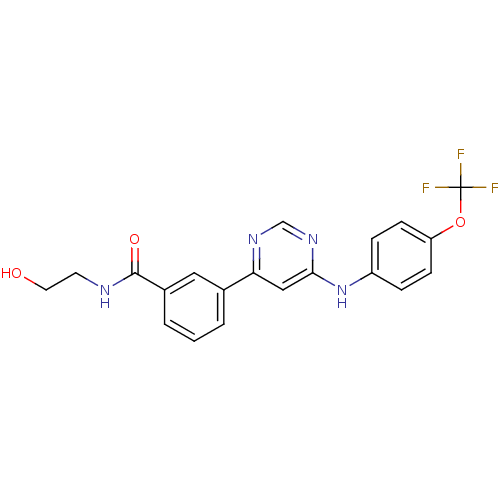
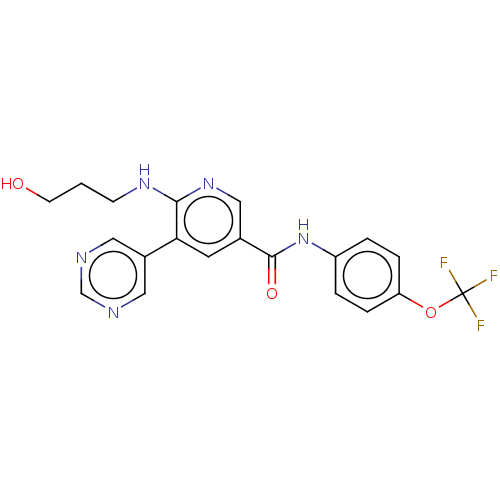
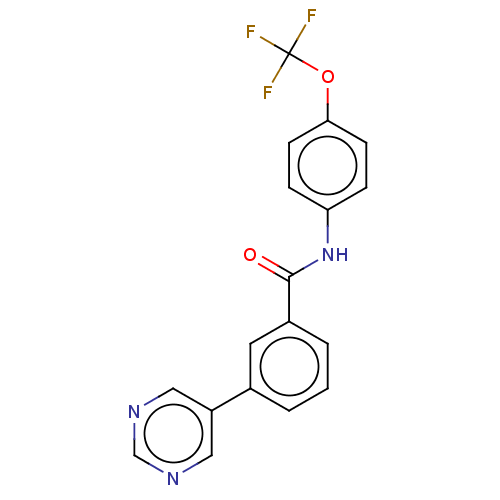
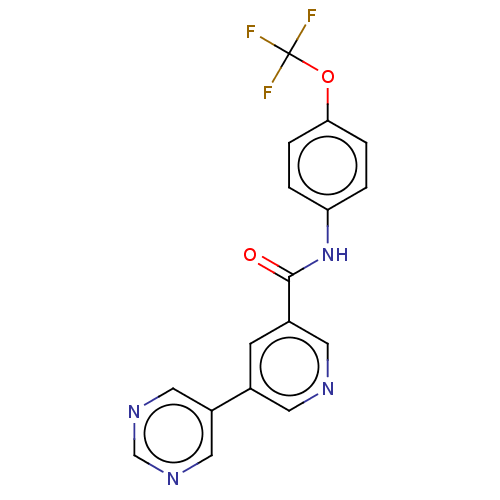
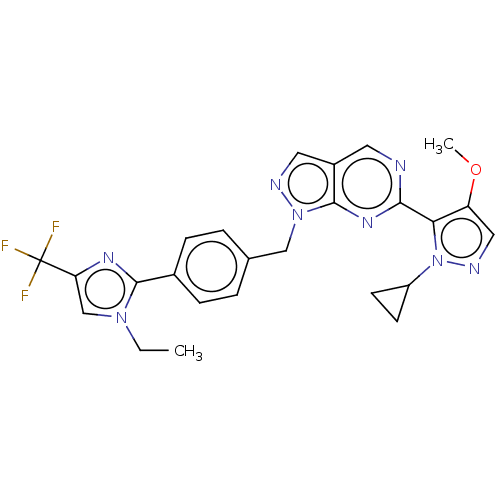
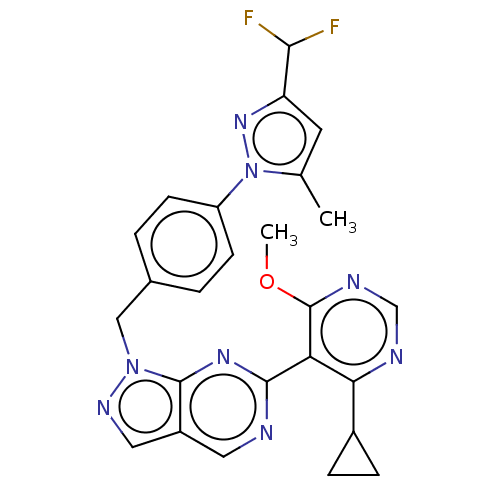
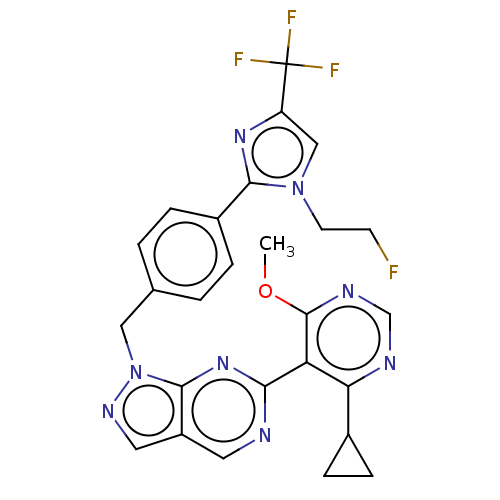
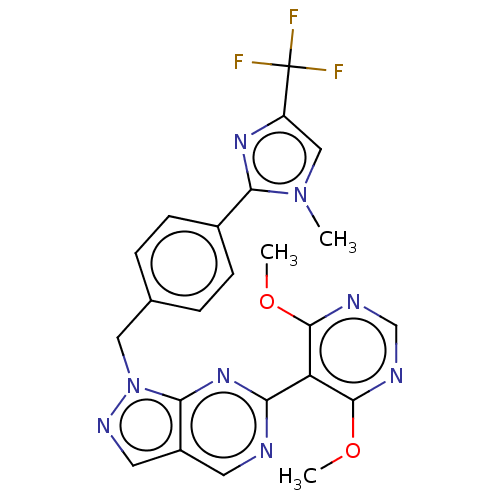
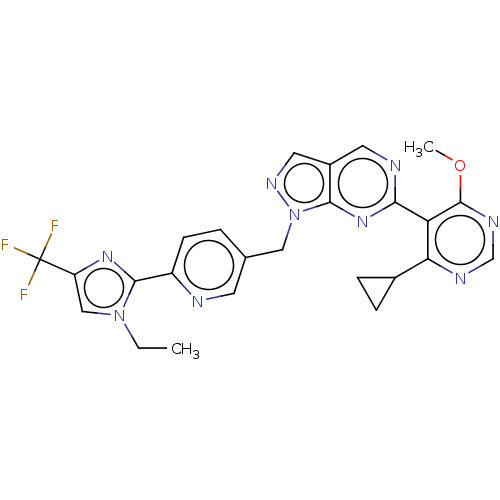
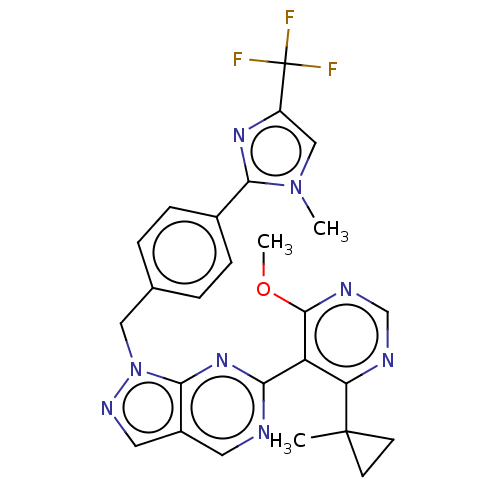
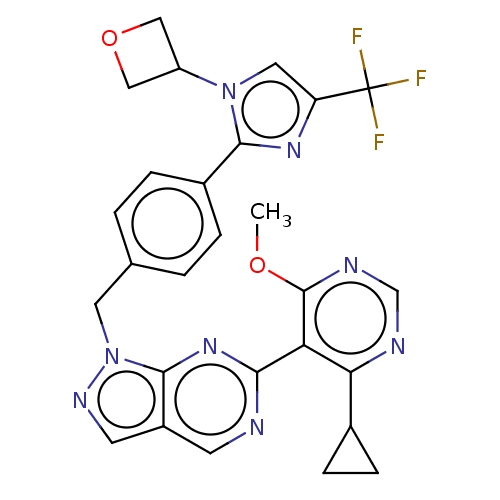
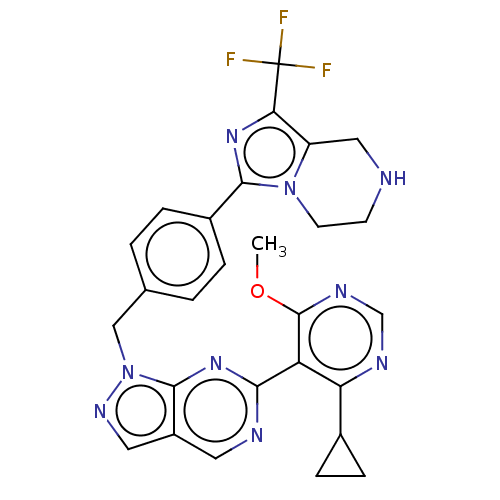
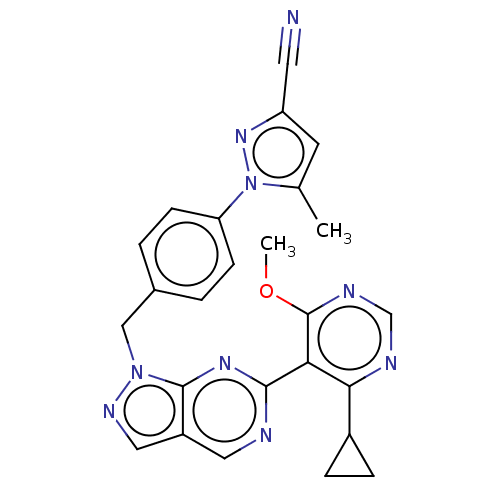
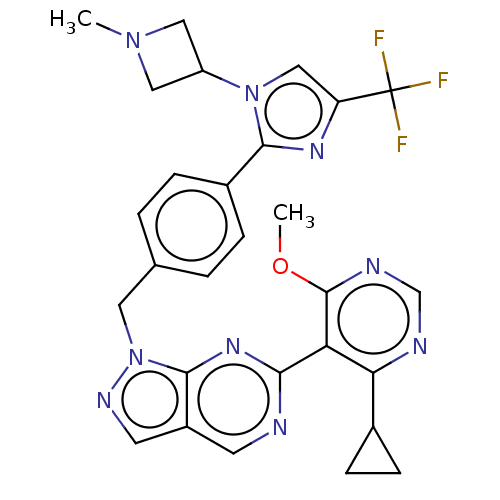
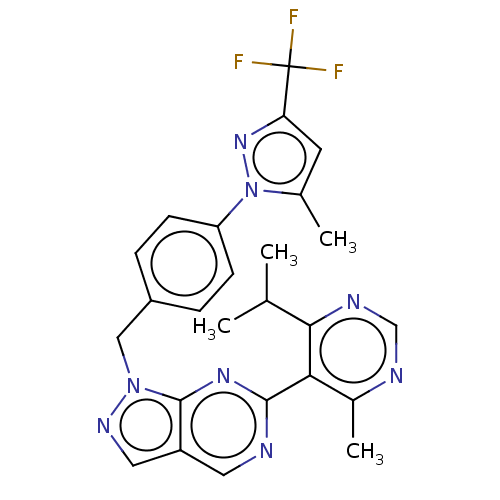
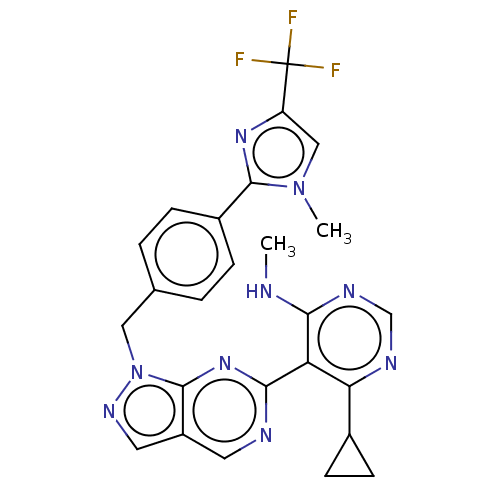
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: <0.300nMAssay Description:Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m...More data for this Ligand-Target Pair
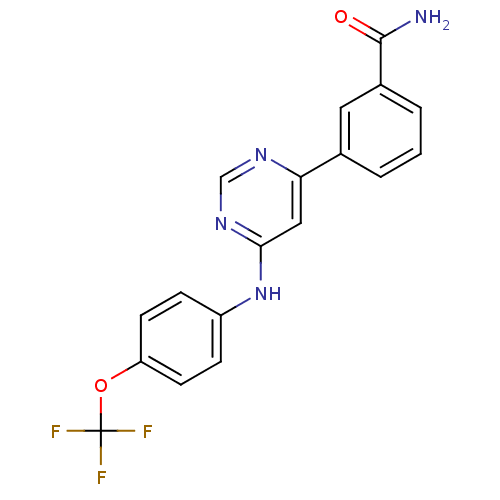
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m...More data for this Ligand-Target Pair
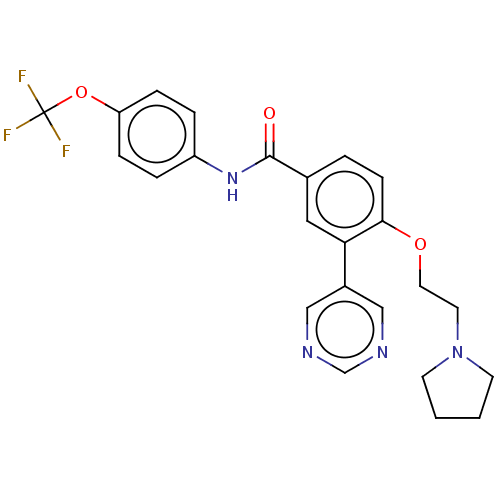
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m...More data for this Ligand-Target Pair
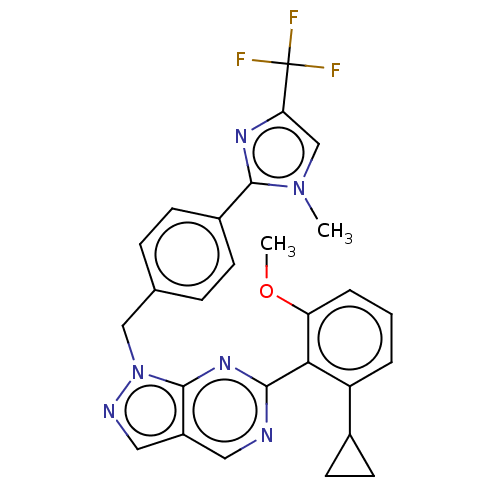
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 0.700nMAssay Description:Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of recombinant human c-ABL SH3/SH2/SH1 domain (46 to 531 residues) expressed in sf9 insect cells after 30 mins in presence of [gamma-32P]A...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Displacement of [3H]dofetilide from human ERG by high throughput assayMore data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: <10nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of recombinant human c-ABL SH3/SH2/SH1 domain (46 to 515 residues) expressed in bacterial expression system using EAIYAAPFAKKK as substrat...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase ABL1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 24nMAssay Description:Inhibition of ABL1 (64 to 515 residues)(unknown origin) expressed in Escherichia coli using FITC-Ahx-EAIYAAPFAKKK-NH2 peptide as substrate after 60 m...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Deubiquitinase activity was measured using ubiquitin-rhodamine 110 as a substrate. Cleavage of the amide bond between rhodamine and the c-terminal gl...More data for this Ligand-Target Pair

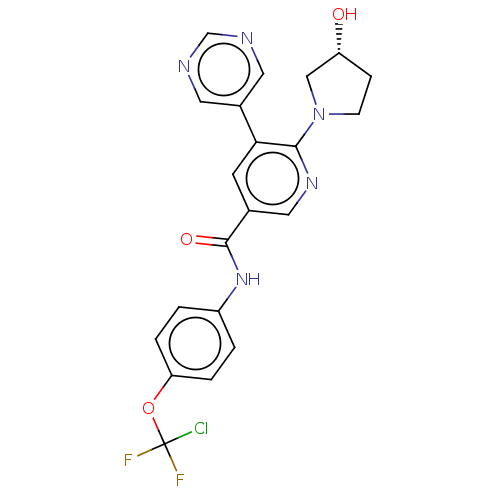
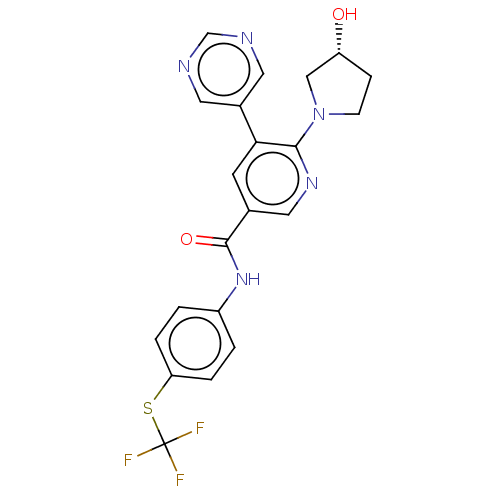
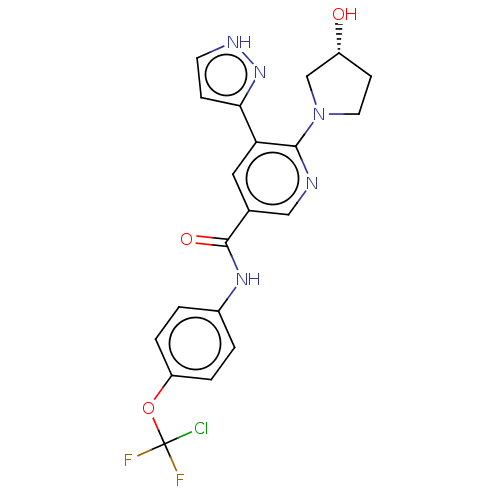
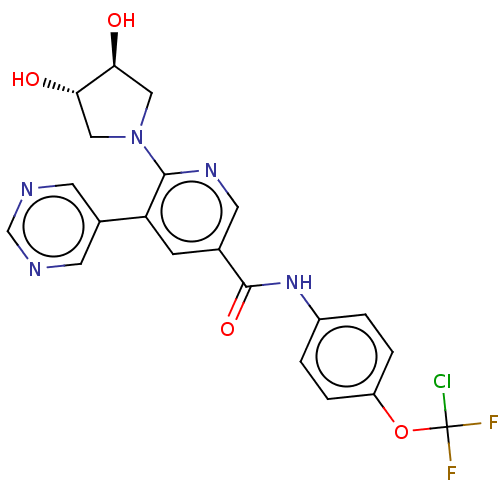

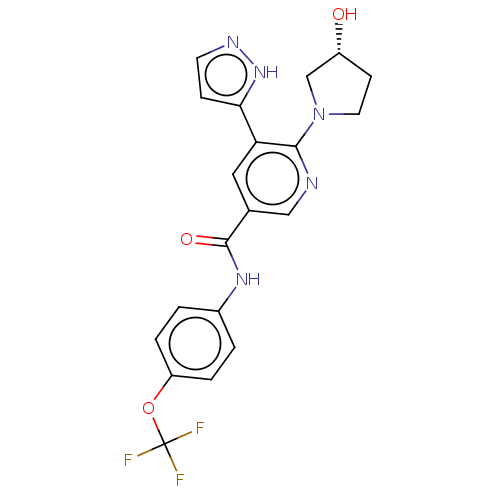
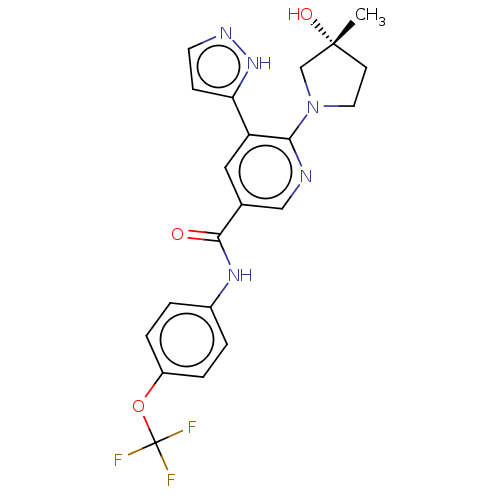
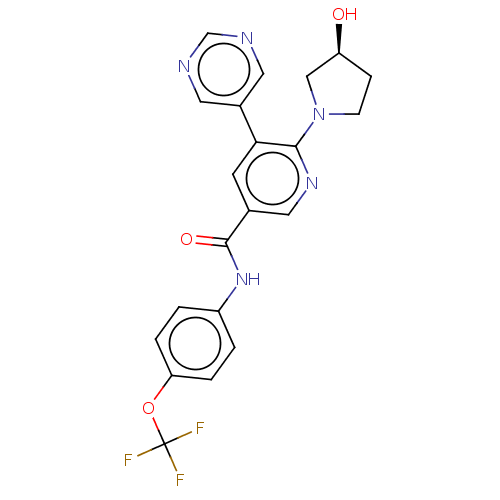
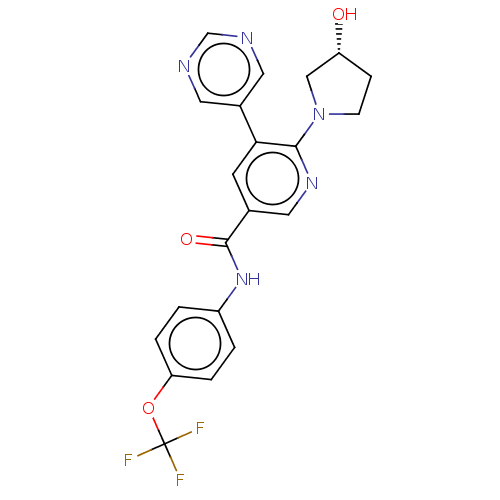
 3D Structure (crystal)
3D Structure (crystal)