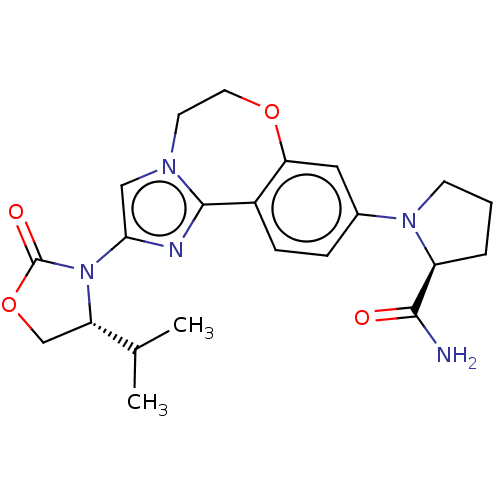
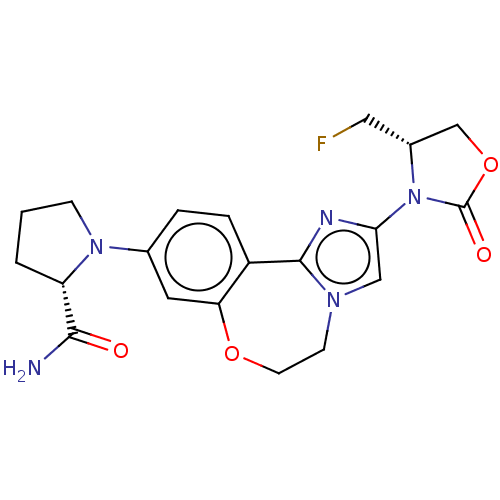
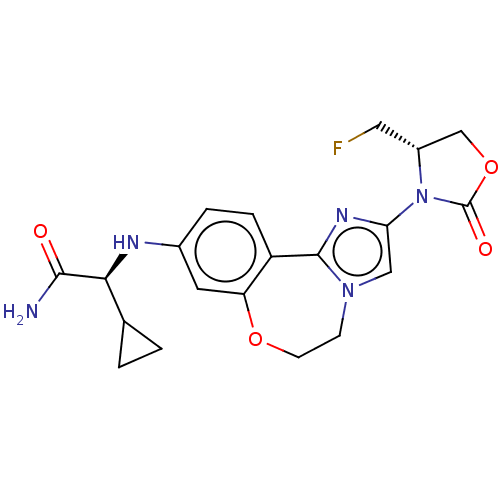
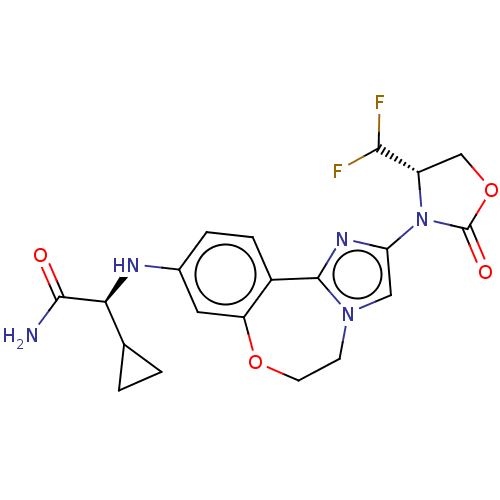
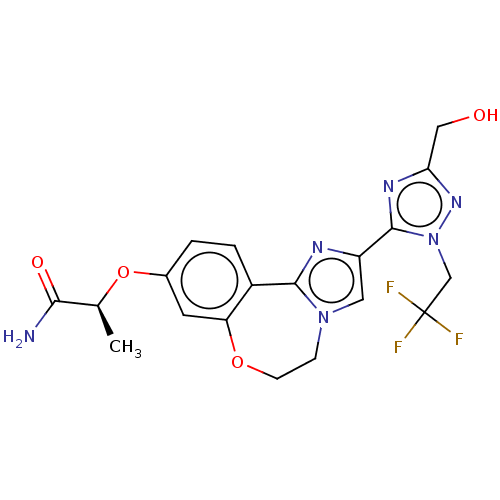
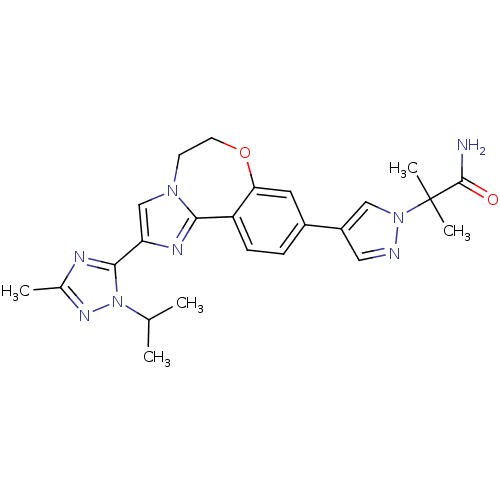
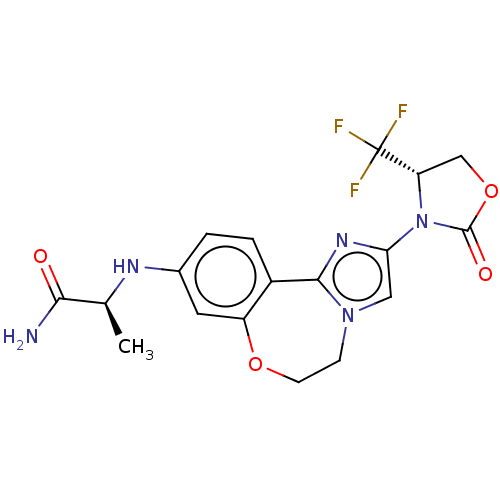
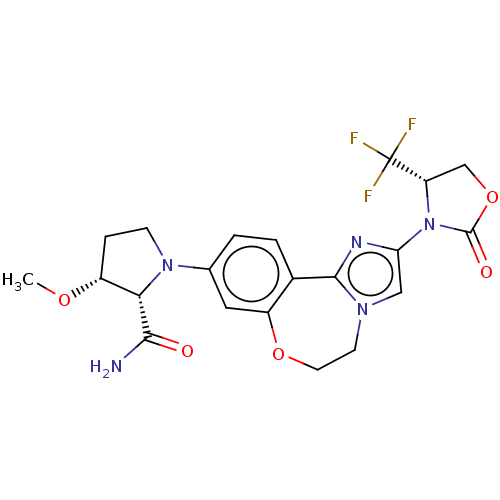
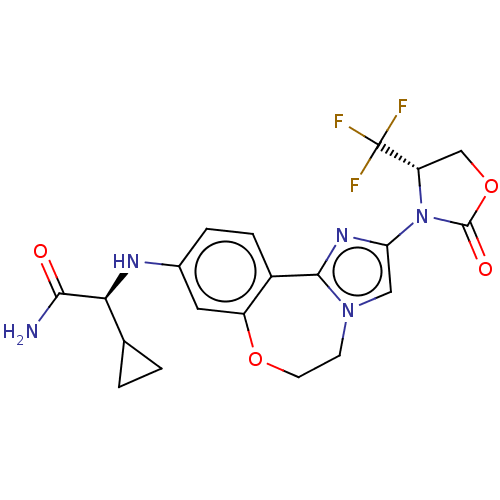
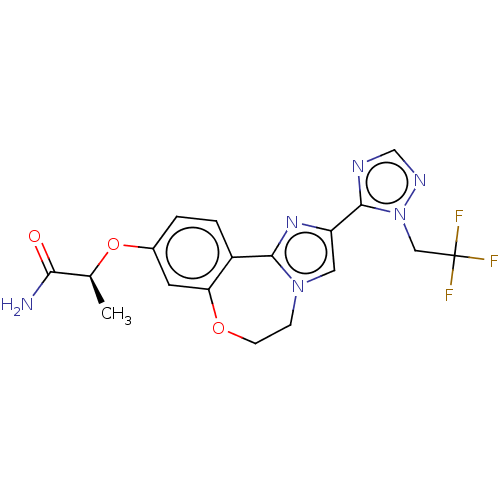
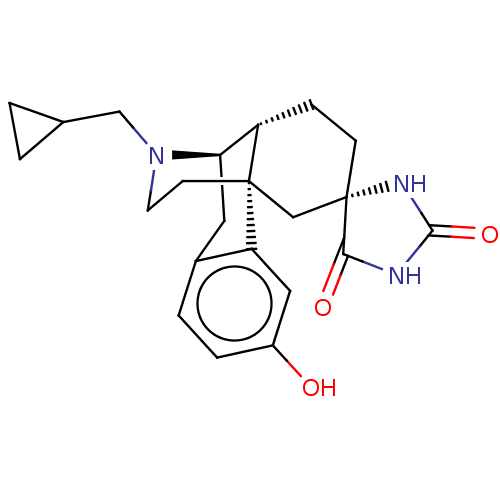
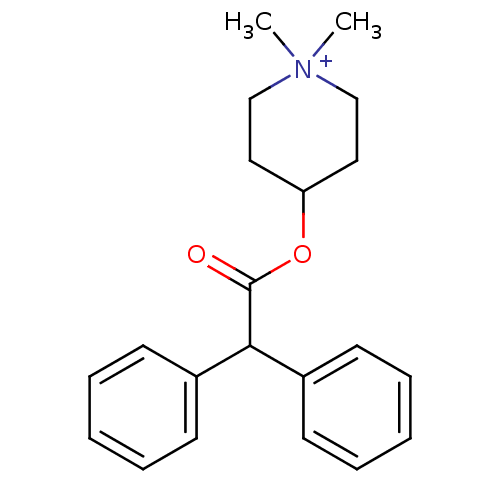
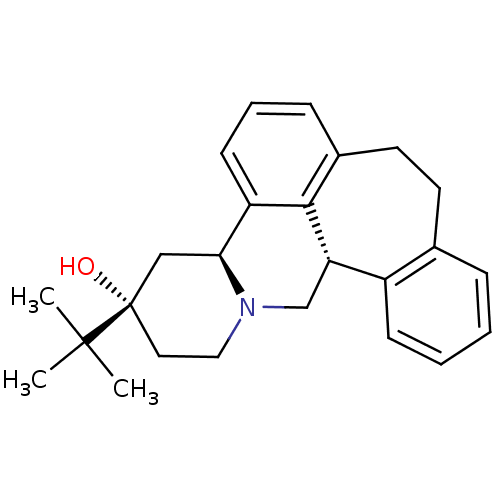
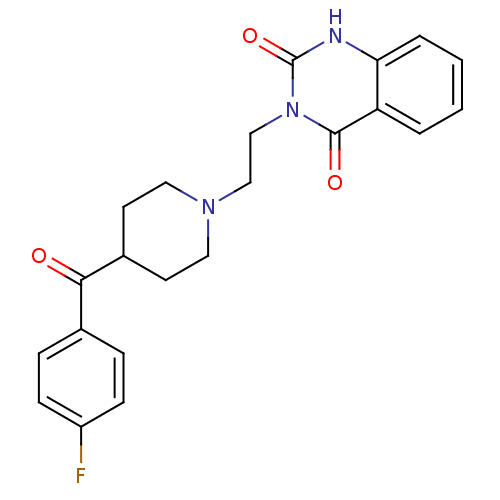
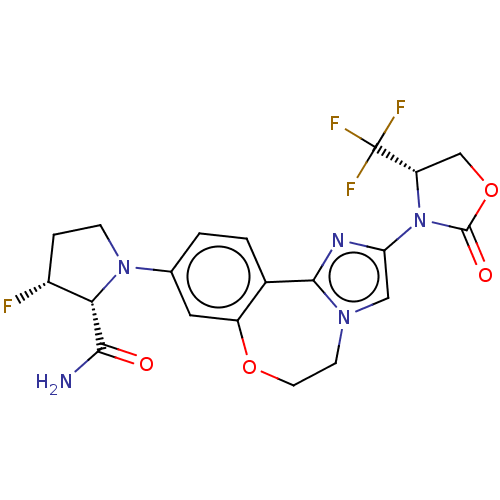
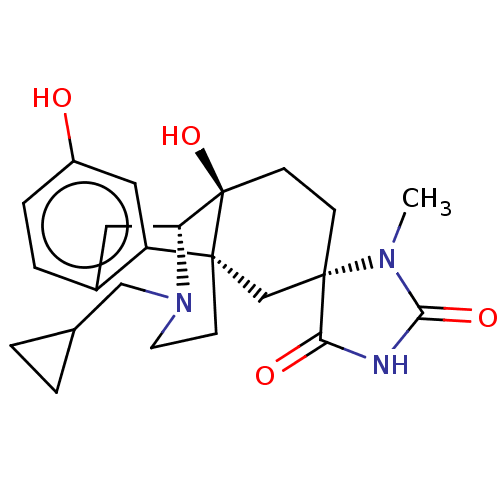
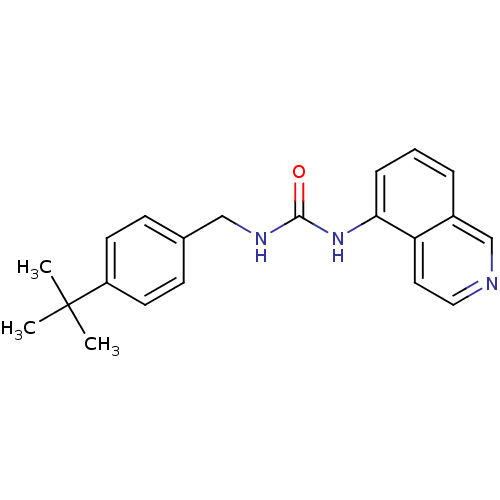
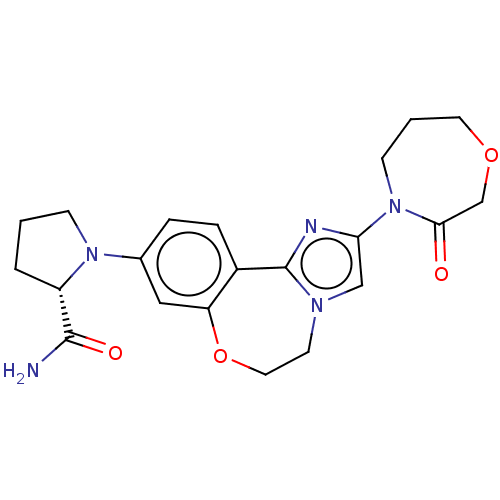
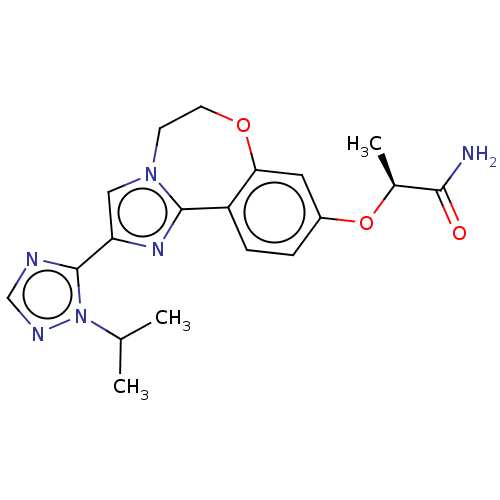
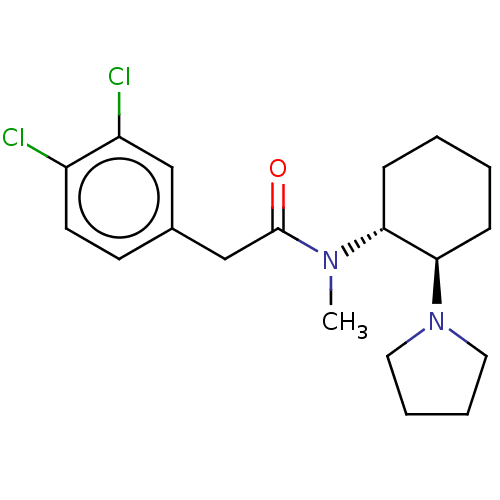
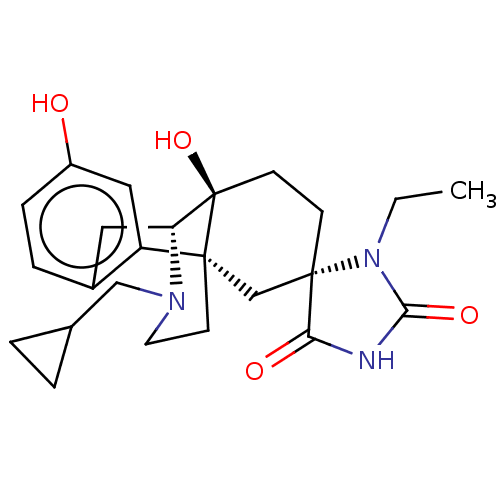
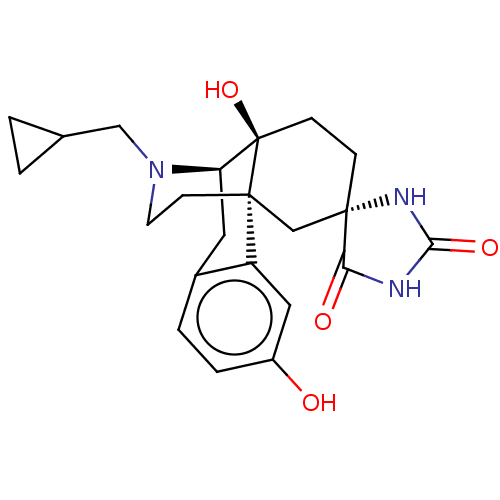
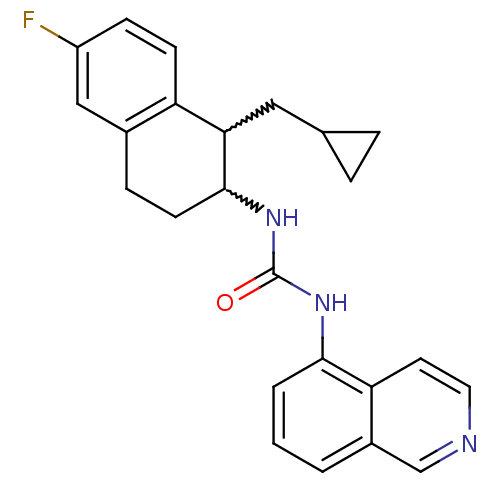
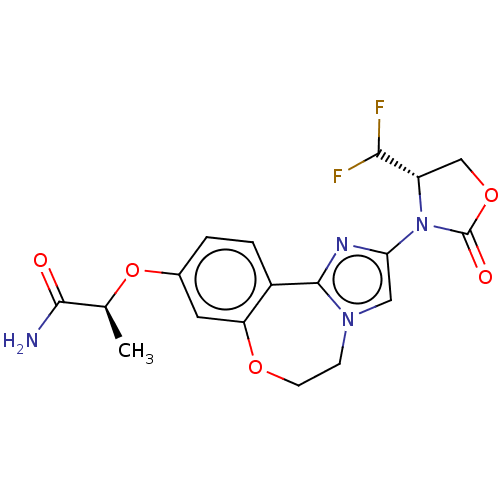
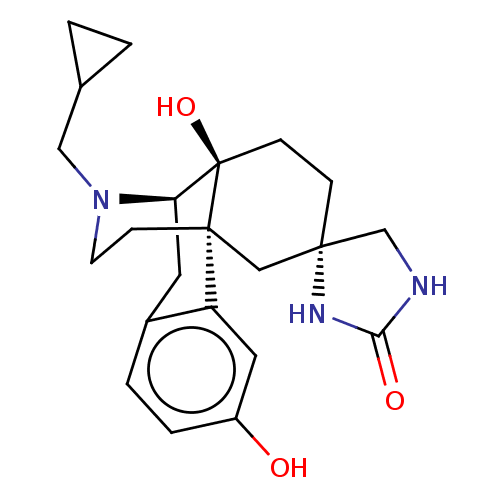
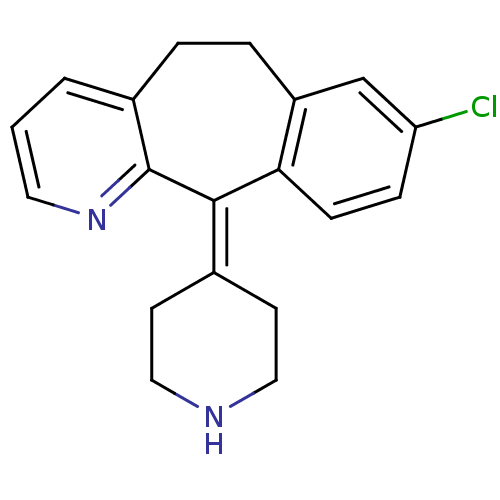
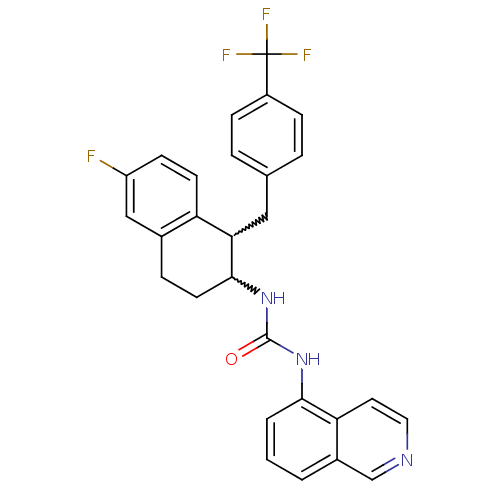
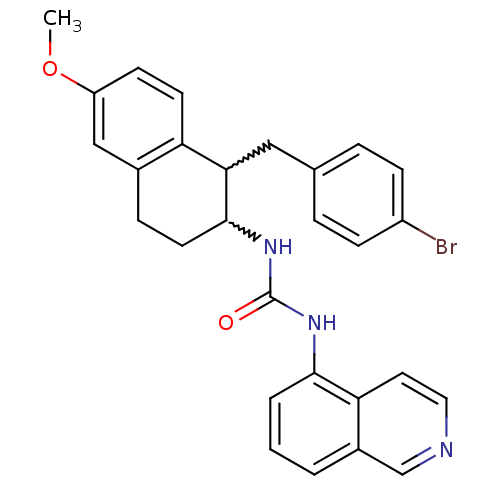
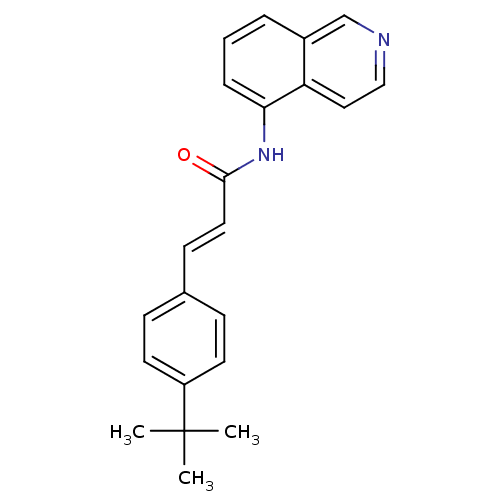
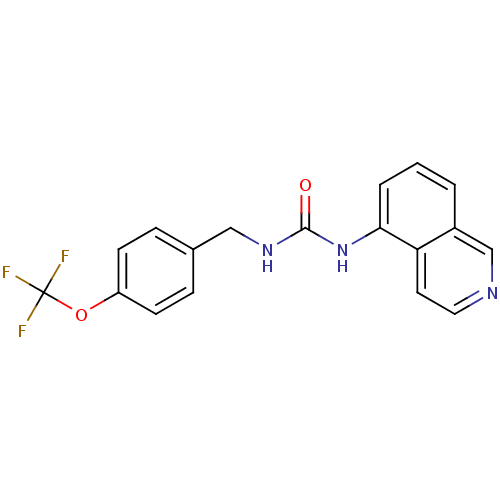
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0260nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
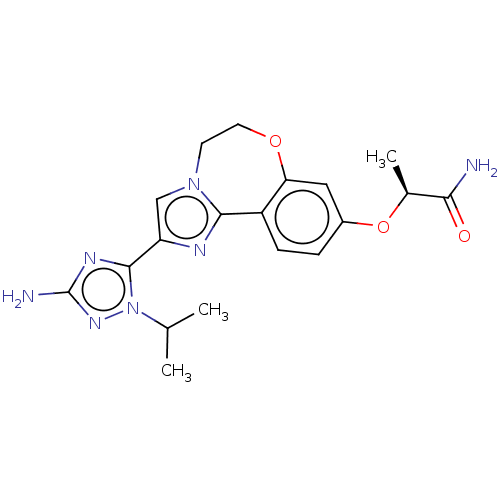
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0340nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
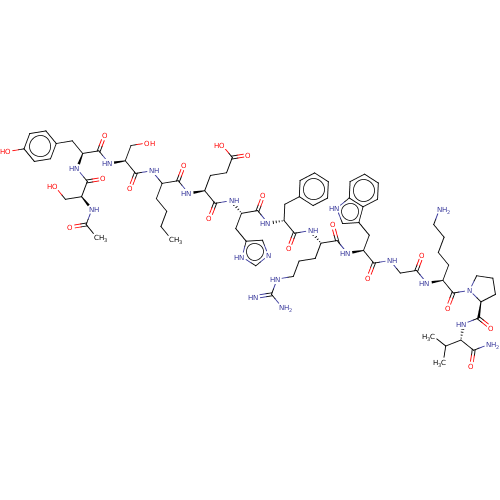
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0420nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
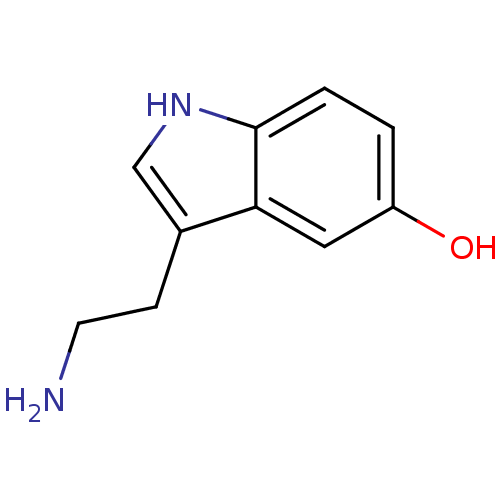
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0430nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0510nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0530nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0600nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0620nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0900nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0950nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.0970nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.100nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.107nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:Inhibition of human recombinant melanocortin 4 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.150nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.157nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
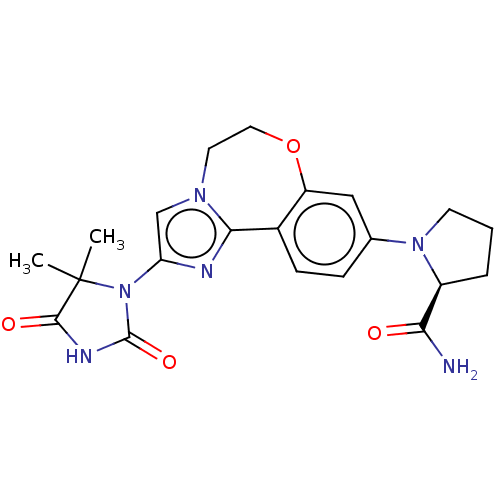
Target5-hydroxytryptamine receptor 7(Homo sapiens (Human))
The University Of Newcastle
Curated by ChEMBL
The University Of Newcastle
Curated by ChEMBL
Affinity DataKi: 0.170nMAssay Description:Displacement of [3H]LSD from human recombinant 5-HT7 receptor expressed in CHO cells after 120 mins by scintillation countingMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.188nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.200nM ΔG°: -55.4kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant...More data for this Ligand-Target Pair
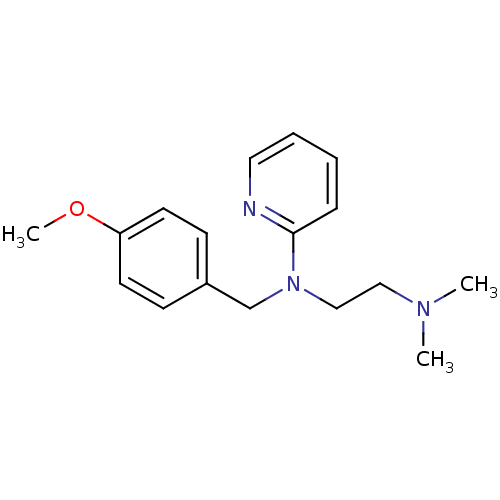
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
The University Of Newcastle
Curated by ChEMBL
The University Of Newcastle
Curated by ChEMBL
Affinity DataKi: 0.220nMAssay Description:Displacement of [3H]4-DAMP from human recombinant Muscarinic acetylcholine receptor M3 expressed in CHO cells after 60 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.230nM ΔG°: -55.0kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant...More data for this Ligand-Target Pair
Affinity DataKi: 0.270nMAssay Description:Displacement of [3H]methyl-spiperone from human recombinant D2S receptor in HEK293 cells after 60 mins by scintillation countingMore data for this Ligand-Target Pair
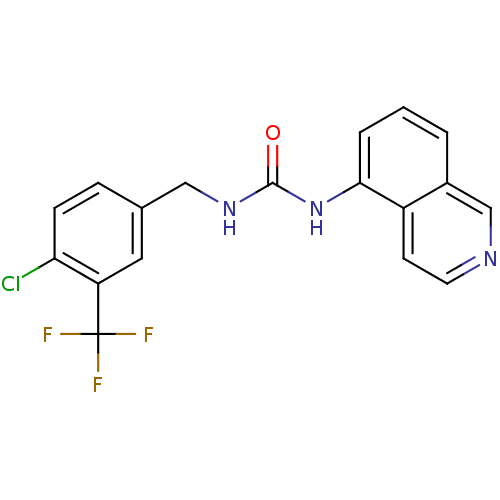
Target5-hydroxytryptamine receptor 2A(Homo sapiens (Human))
The University Of Newcastle
Curated by ChEMBL
The University Of Newcastle
Curated by ChEMBL
Affinity DataKi: 0.280nMAssay Description:Displacement of [3H]ketanserin from human recombinant 5-HT2A receptor in HEK293 cells after 60 mins by scintillation countingMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.289nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nM ΔG°: -54.4kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant...More data for this Ligand-Target Pair
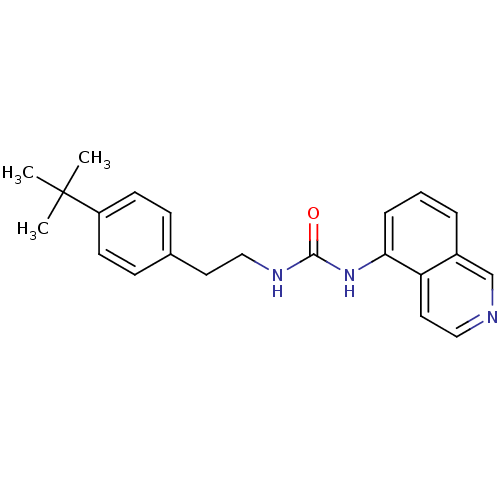
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand.More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.320nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.323nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.346nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.355nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.440nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Rattus norvegicus (rat))
The University Of Newcastle
Curated by ChEMBL
The University Of Newcastle
Curated by ChEMBL
Affinity DataKi: 0.450nMAssay Description:Displacement of [3H]U 69593 from rat recombinant kappa opioid receptor expressed in CHO cells after 60 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 0.470nM ΔG°: -53.2kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.560nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nM ΔG°: -52.6kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.681nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.692nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.710nMAssay Description:Displacement of [3H]pyrilamine from human recombinant histamine H1 receptor in HEK293 cells after 60 mins by scintillation countingMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand.More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.853nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.860nM ΔG°: -56.1kJ/molepH: 7.4 T: 2°CAssay Description:mu-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement binding assays for mu-opioid receptors used 0.3 nM [3H]-diprenorphine (Per...More data for this Ligand-Target Pair
Affinity DataKi: 0.940nM ΔG°: -51.5kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant...More data for this Ligand-Target Pair
Affinity DataKi: 0.970nMAssay Description:Binding affinity towards human histamine H1 receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Antagonistic activity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane, as inhibition of agonist-induced intracellular [C...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand.More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand.More data for this Ligand-Target Pair

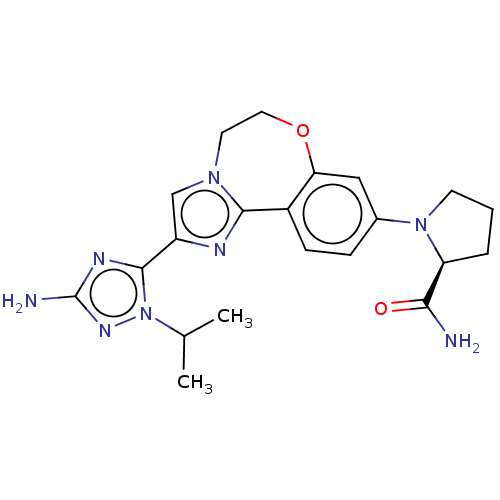
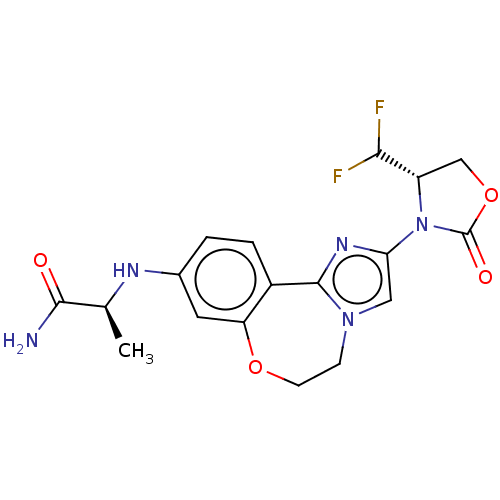
 3D Structure (crystal)
3D Structure (crystal)