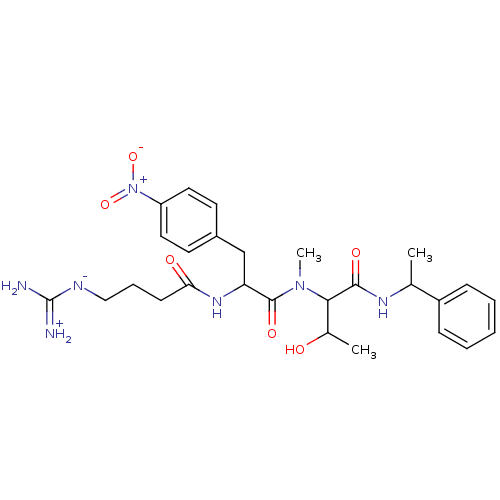
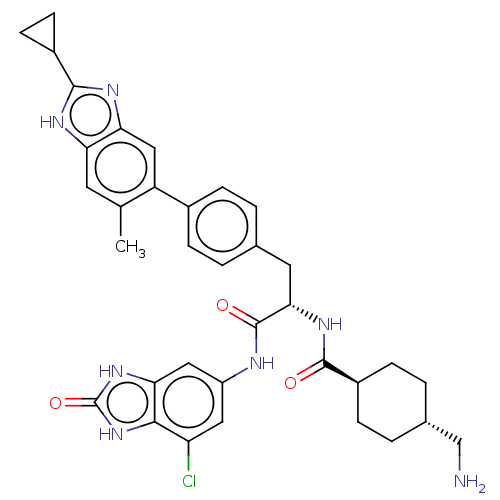
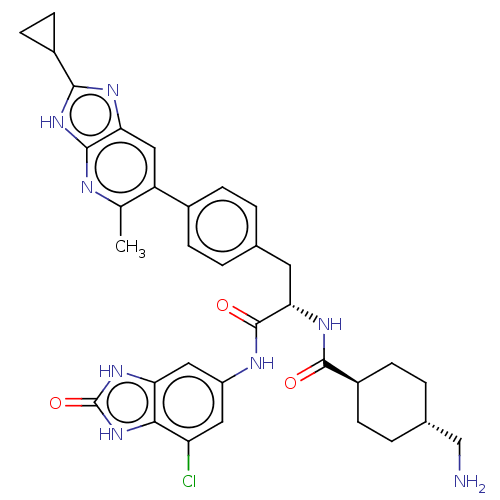
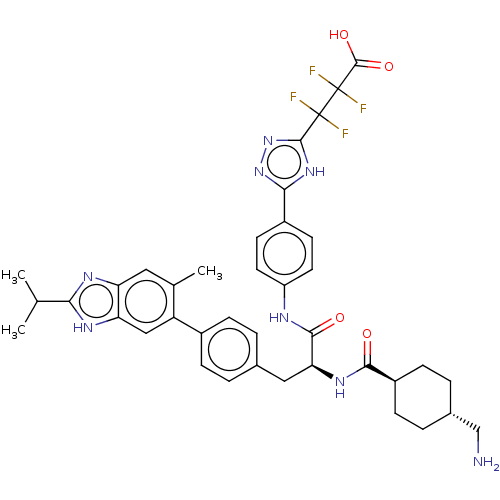
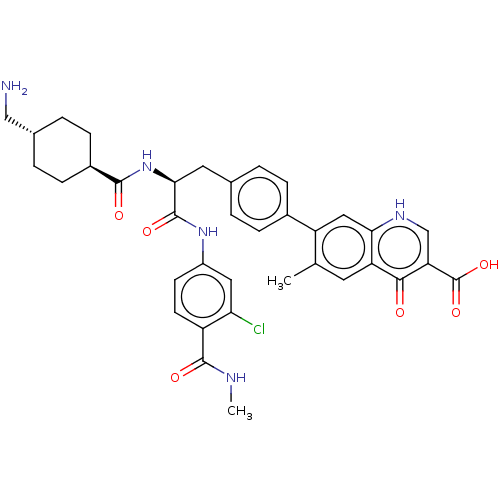
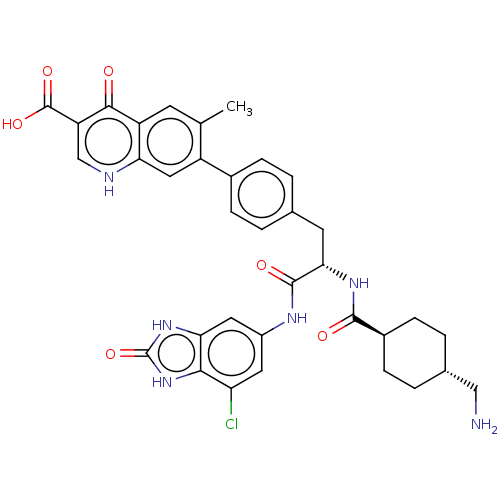
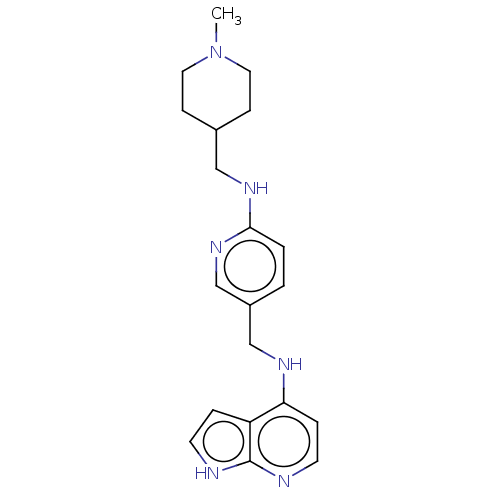
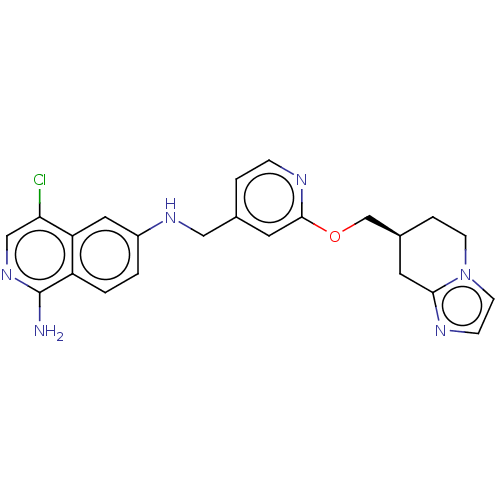
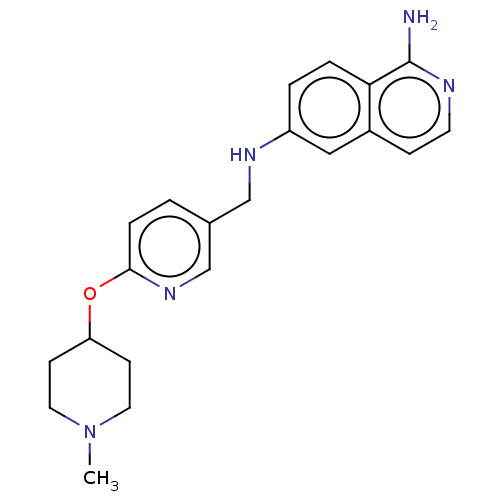
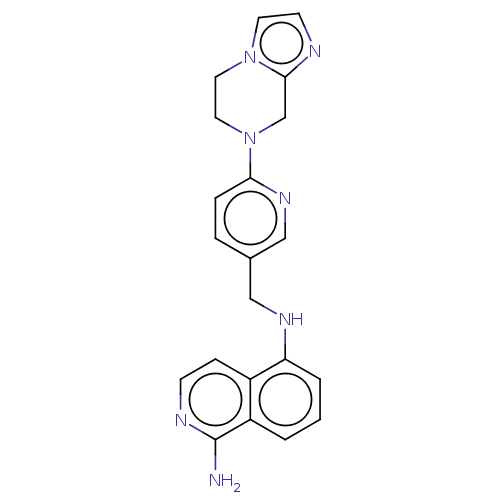
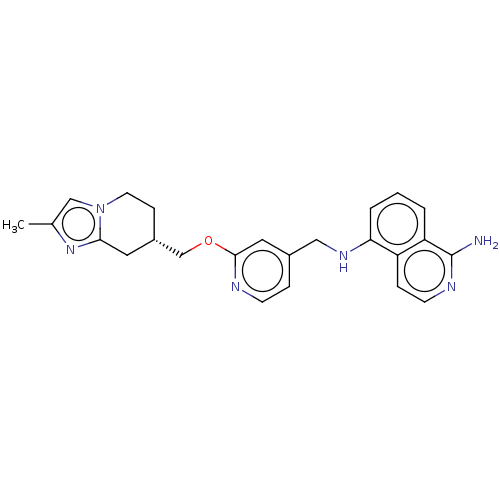
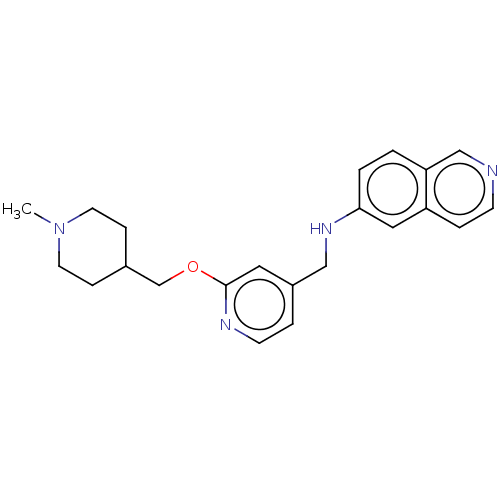
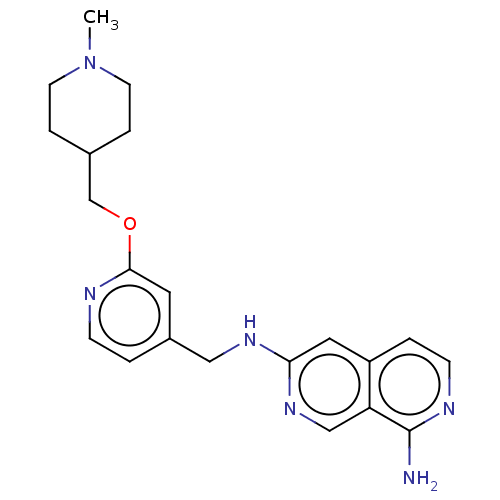
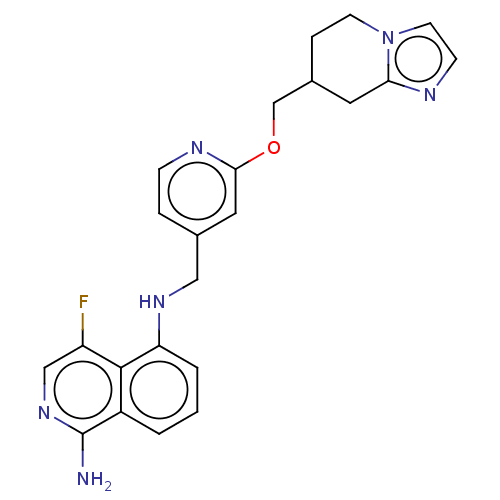
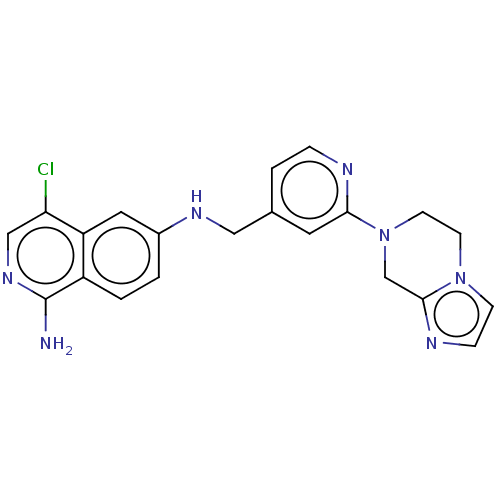
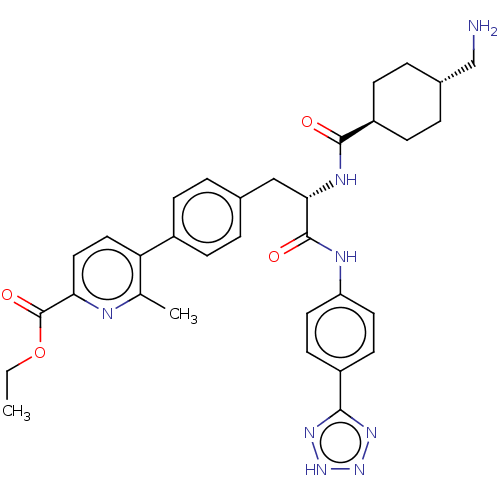
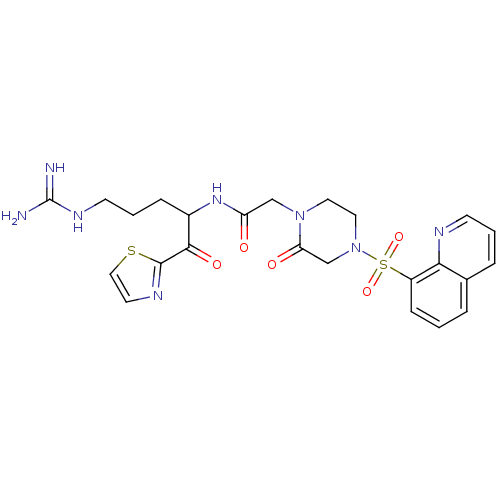
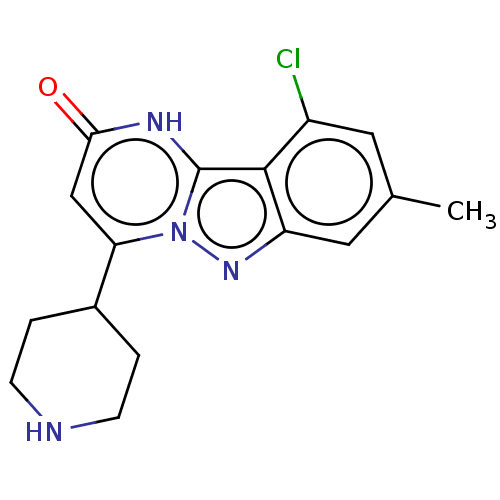
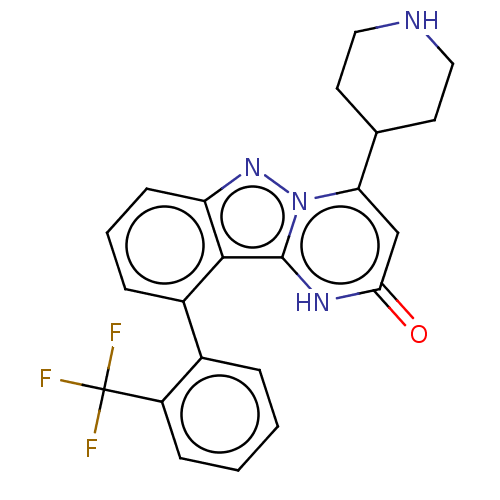
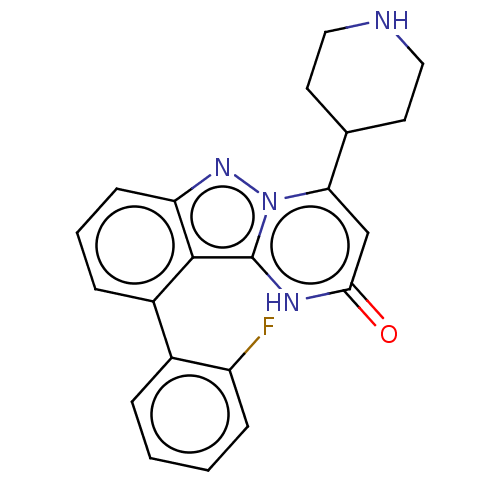
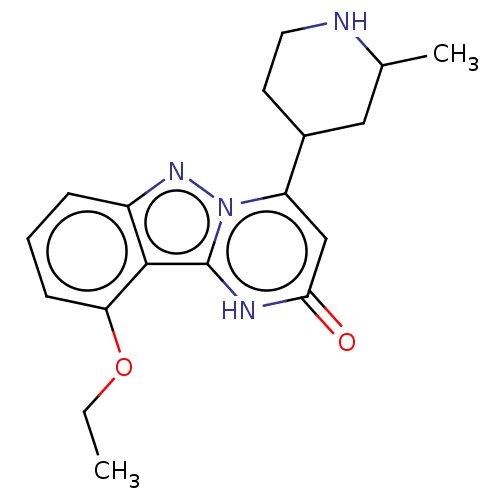
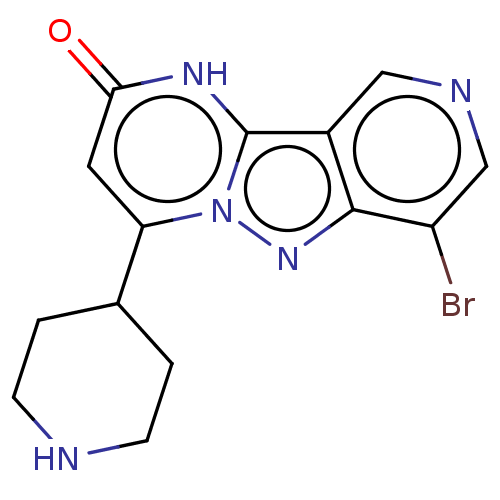
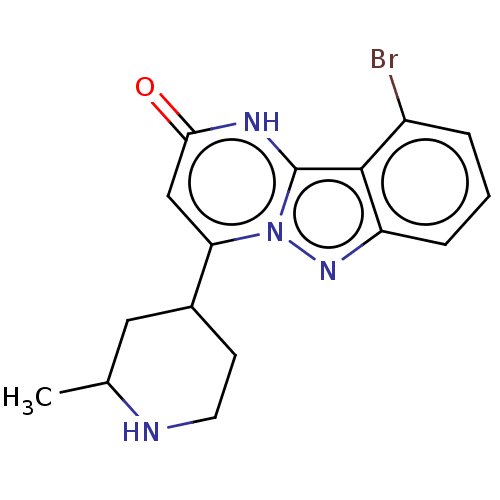
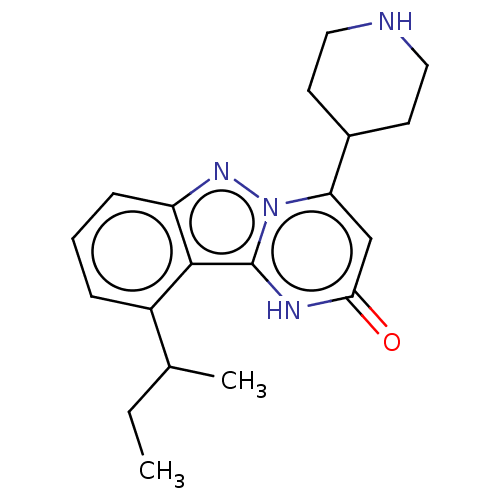
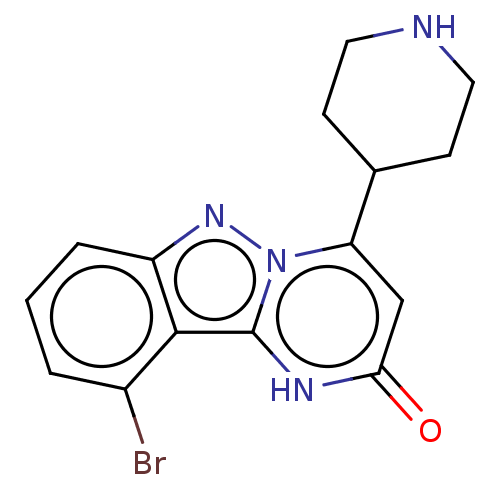
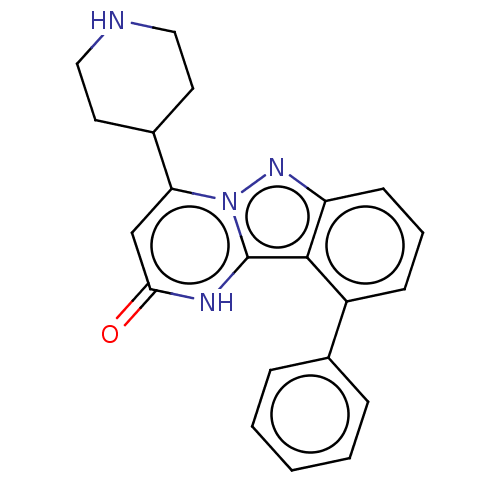
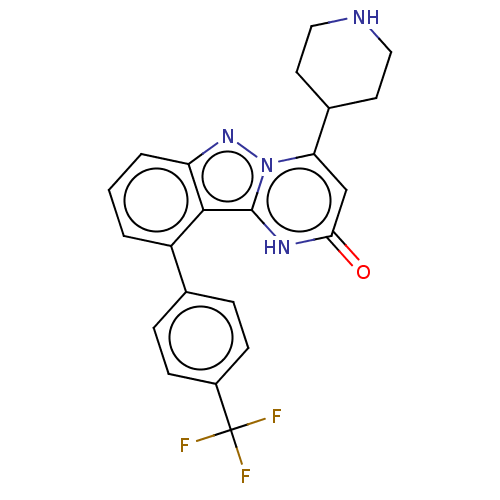
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.230nMAssay Description:Concentration of the compound required to inhibit Plasmin was determinedMore data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.320nMAssay Description:Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence...More data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence...More data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence...More data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.420nMAssay Description:Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence...More data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence...More data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.870nMAssay Description:Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence...More data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence...More data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence...More data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of related proteases was determined using an (C50 assay based on standard literature methods (see e.g. Shori et al., Biochem. Pha...More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of related proteases was determined using an (C50 assay based on standard literature methods (see e.g. Shori et al., Biochem. Pha...More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of related proteases was determined using an (C50 assay based on standard literature methods (see e.g. Shori et al., Biochem. Pha...More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of related proteases was determined using an (C50 assay based on standard literature methods (see e.g. Shori et al., Biochem. Pha...More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of related proteases was determined using an (C50 assay based on standard literature methods (see e.g. Shori et al., Biochem. Pha...More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of related proteases was determined using an (C50 assay based on standard literature methods (see e.g. Shori et al., Biochem. Pha...More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of related proteases was determined using an (C50 assay based on standard literature methods (see e.g. Shori et al., Biochem. Pha...More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of related proteases was determined using an (C50 assay based on standard literature methods (see e.g. Shori et al., Biochem. Pha...More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of related proteases was determined using an (C50 assay based on standard literature methods (see e.g. Shori et al., Biochem. Pha...More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of related proteases was determined using an (C50 assay based on standard literature methods (see e.g. Shori et al., Biochem. Pha...More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of related proteases was determined using an (C50 assay based on standard literature methods (see e.g. Shori et al., Biochem. Pha...More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of related proteases was determined using an (C50 assay based on standard literature methods (see e.g. Shori et al., Biochem. Pha...More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of related proteases was determined using an (C50 assay based on standard literature methods (see e.g. Shori et al., Biochem. Pha...More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:In vitro inhibition of related proteases was determined using an (C50 assay based on standard literature methods (see e.g. Shori et al., Biochem. Pha...More data for this Ligand-Target Pair
Target InfoPDBMMDBNCI pathwayReactome pathwayKEGG
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
UniProtKB/SwissProt
B.MOADDrugBankantibodypediaGoogleScholar
Ligand Info
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 8.60nMAssay Description:Inhibition of human plasmin preincubated for 15 mins followed by MeOSuc-Ala-Phe-Lys-AMC substrate addition and measured after 30 mins by fluorescence...More data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 9.60nMAssay Description:In vitro Enzyme Inhibitory activity measured against PlasminMore data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: >10nMAssay Description:Inhibition of plasmin (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
TargetPlasminogen(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibitory activity against PlasminMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.0Assay Description:The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMpH: 7.0Assay Description:The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMpH: 7.0Assay Description:The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 7.0Assay Description:The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 7.0Assay Description:The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 7.0Assay Description:The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 7.0Assay Description:The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
Affinity DataIC50: 13nMpH: 7.0Assay Description:The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMpH: 7.0Assay Description:The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMpH: 7.0Assay Description:The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMpH: 7.0Assay Description:The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMpH: 7.0Assay Description:The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMpH: 7.0Assay Description:The test compounds were dissolved in 1% acetic acid and further complemented with an equal volume of DMSO. The resulting stock solutions were seriall...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:The clot-lysis test system configures the kinetics of clot formation and degradation in vitro and allows quantifying modulation of the process by sel...More data for this Ligand-Target Pair