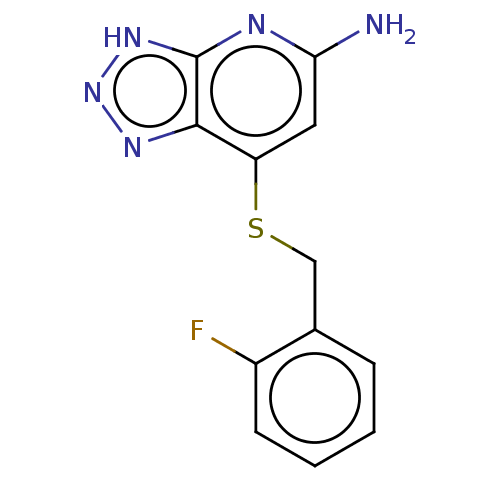
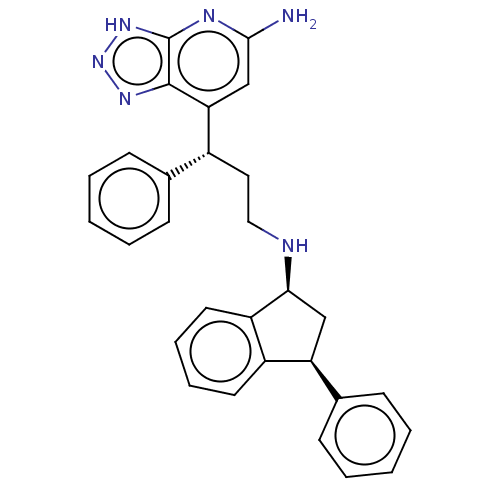
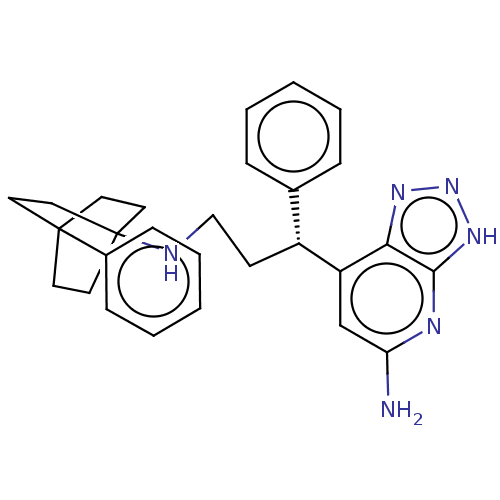
Affinity DataIC50: 17nMAssay Description:Inhibition of human EPX bromination activity assessed as reduction in H2O2 catalyzed 3-bromo tyrosine formation from tyrosine and potassium bromide p...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of human EPX bromination activity assessed as reduction in H2O2 catalyzed 3-bromo tyrosine formation from tyrosine and potassium bromide p...More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of human EPX bromination activity using tyrosine as substrate by measuring 3-bromo tyrosine formation incubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:EPX bromination activity was measured in 100 mM KPi (pH 7.4) by monitoring the H2O2 catalyzed formation of 3-bromo tyrosine from tyrosine and potassi...More data for this Ligand-Target Pair
Affinity DataIC50: 63nMAssay Description:EPX bromination activity was measured in 100 mM KPi (pH 7.4) by monitoring the H2O2 catalyzed formation of 3-bromo tyrosine from tyrosine and potassi...More data for this Ligand-Target Pair
Affinity DataIC50: 92nMAssay Description:Inhibition of human EPX bromination activity using tyrosine as substrate by measuring 3-bromo tyrosine formation incubated for 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:EPX bromination activity was measured in 100 mM KPi (pH 7.4) by monitoring the H2O2 catalyzed formation of 3-bromo tyrosine from tyrosine and potassi...More data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:EPX bromination activity was measured in 100 mM KPi (pH 7.4) by monitoring the H2O2 catalyzed formation of 3-bromo tyrosine from tyrosine and potassi...More data for this Ligand-Target Pair
Affinity DataIC50: 360nMAssay Description:Inhibition of human EPX bromination activity using tyrosine as substrate by measuring 3-bromo tyrosine formation incubated for 10 minsMore data for this Ligand-Target Pair