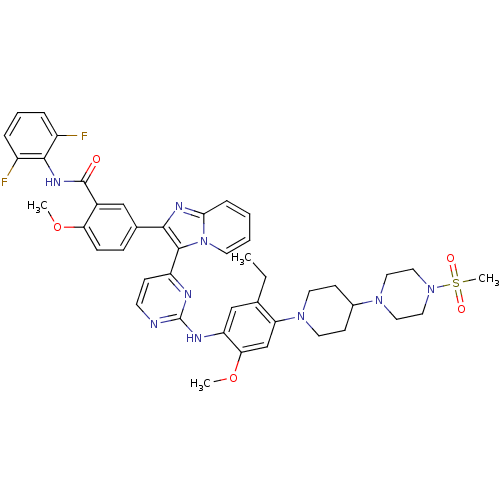
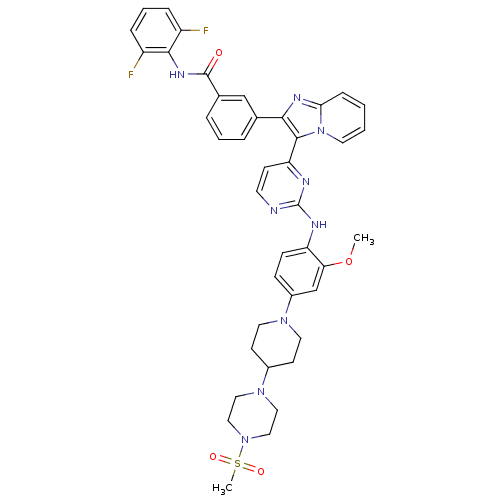
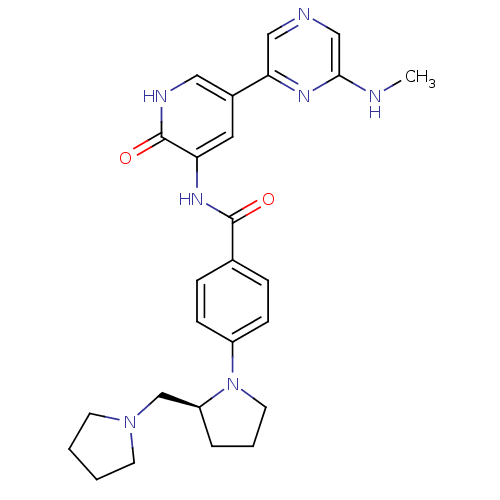
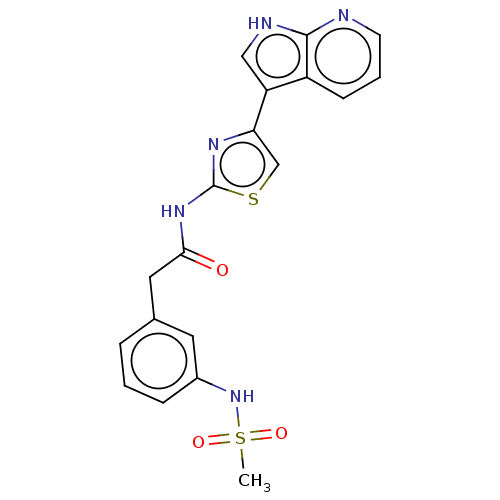
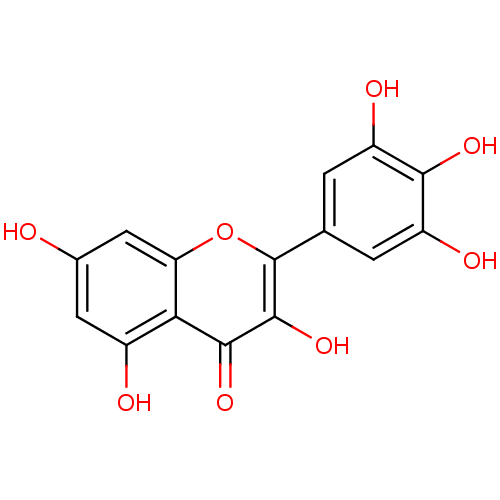
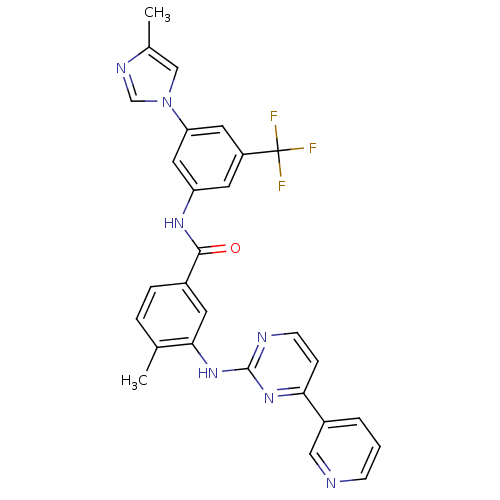
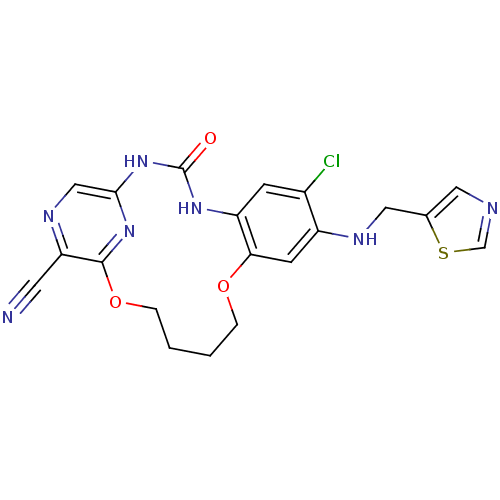
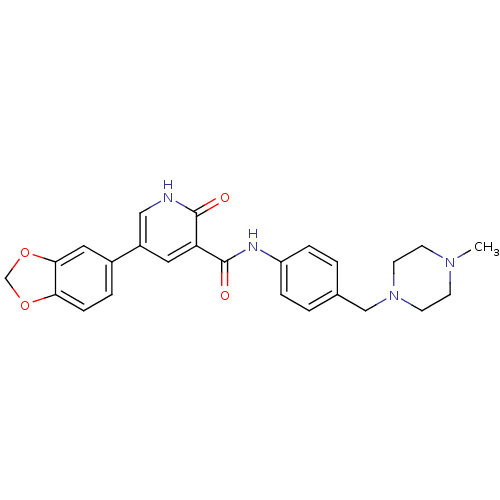
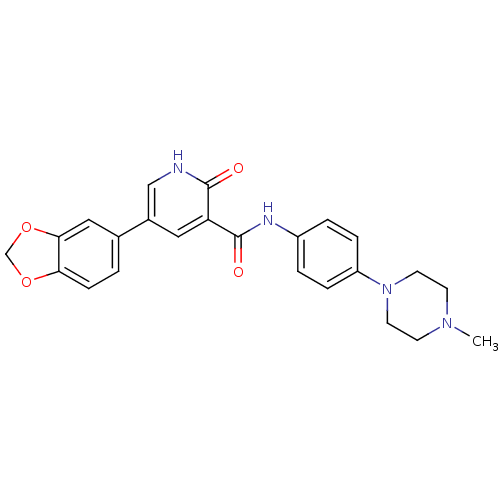
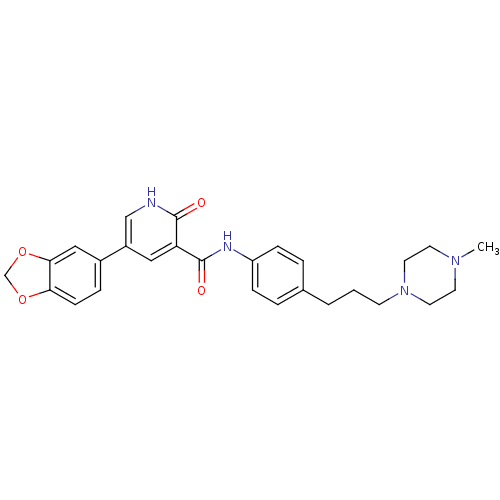
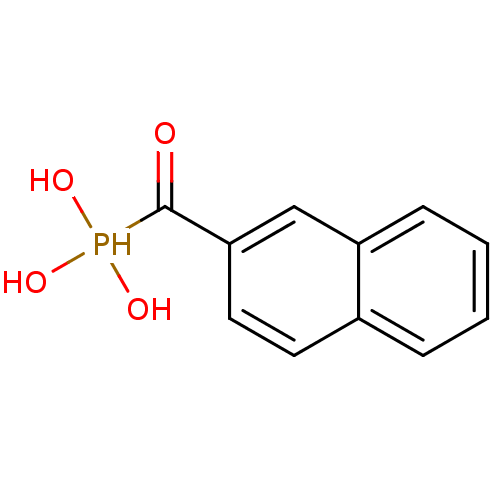
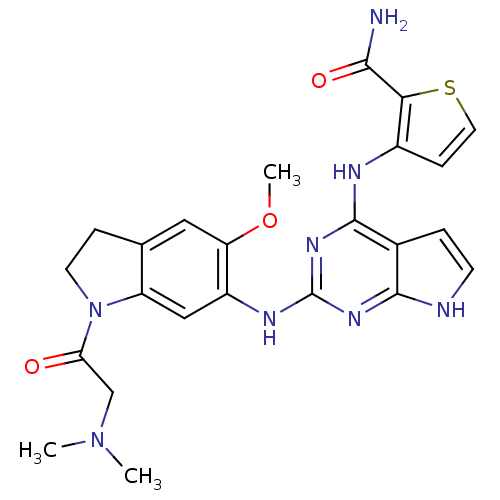
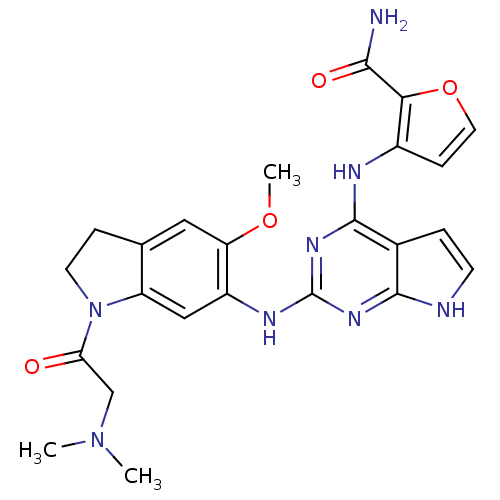
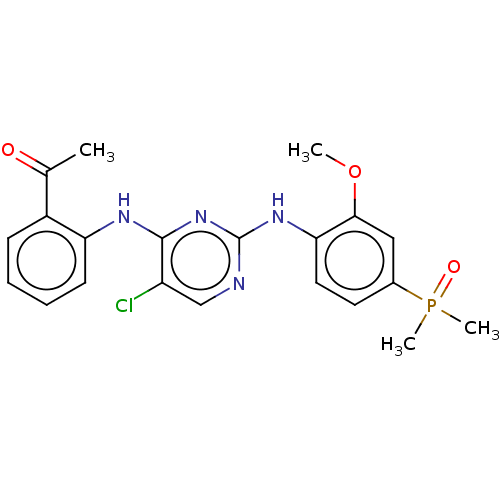
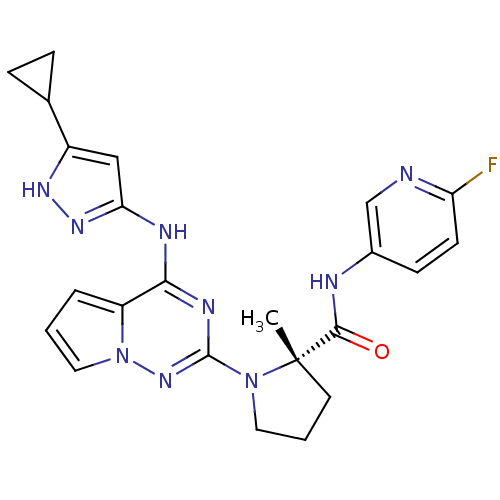
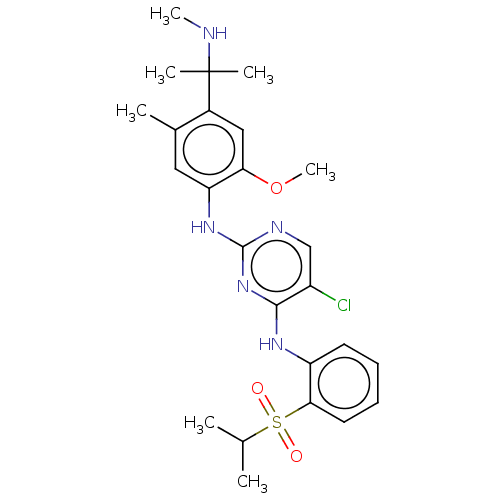
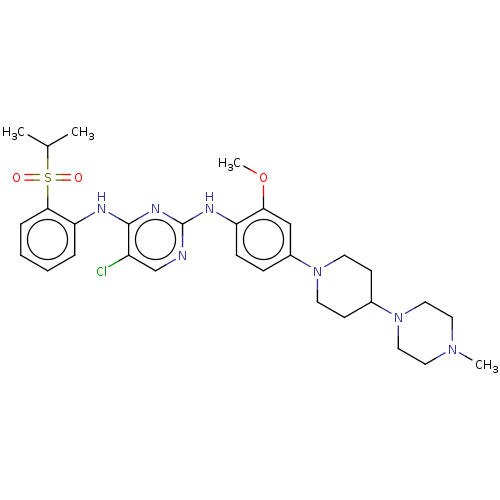
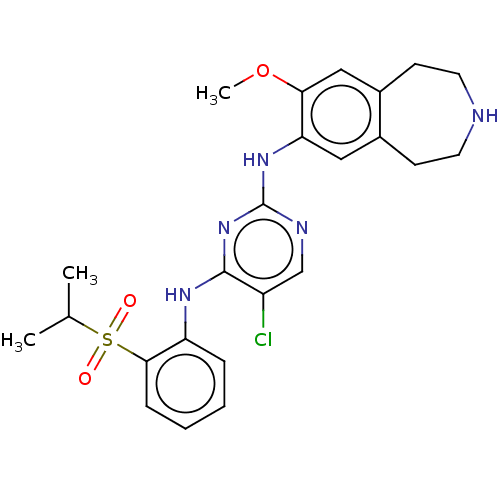
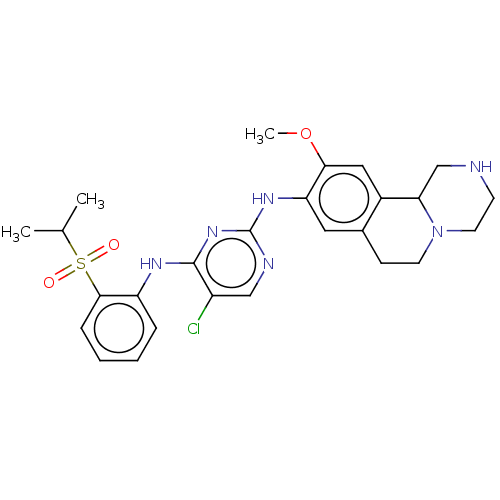
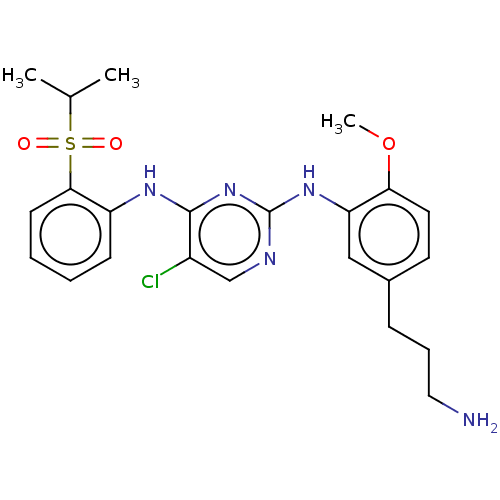
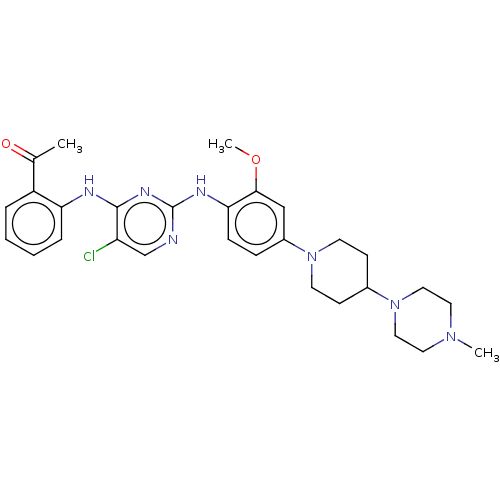
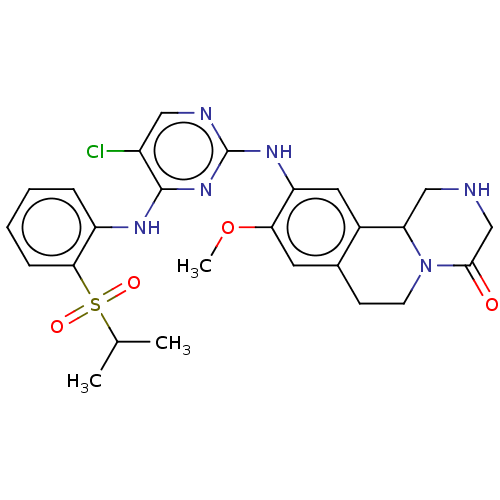
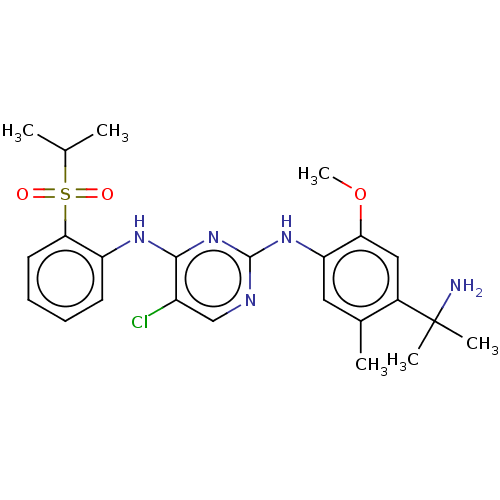
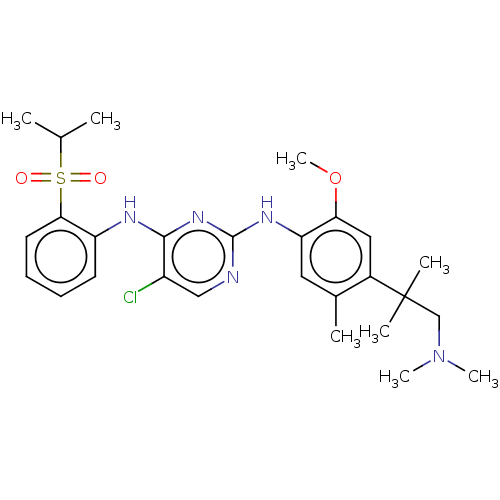
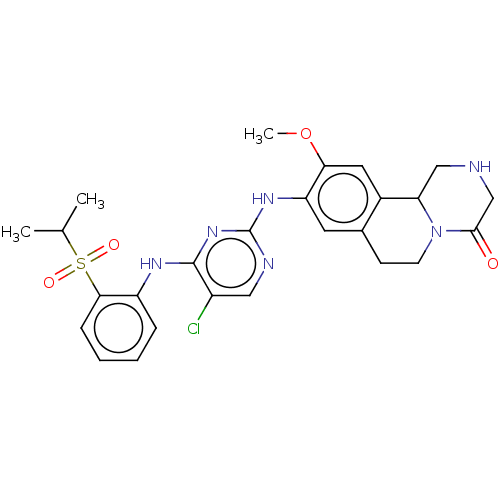
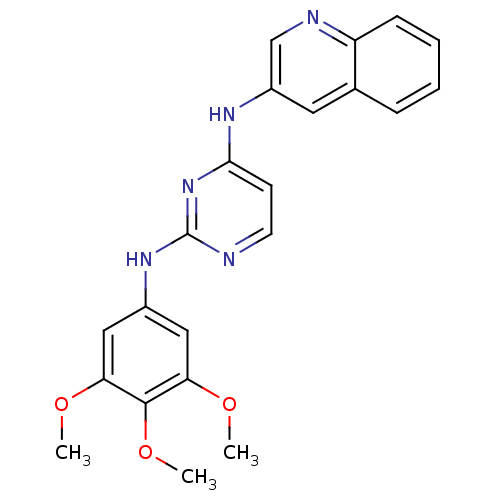
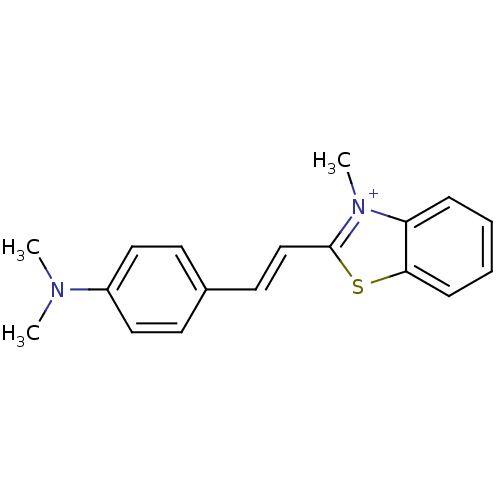
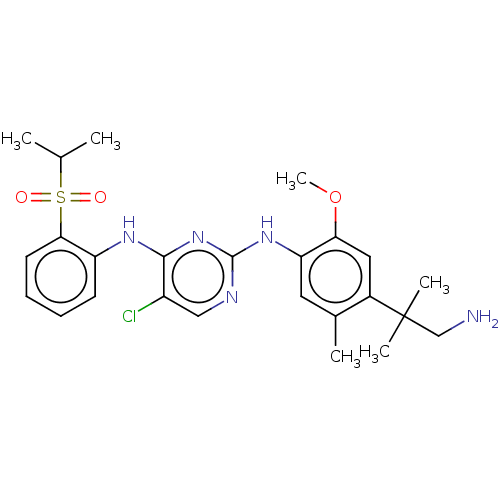
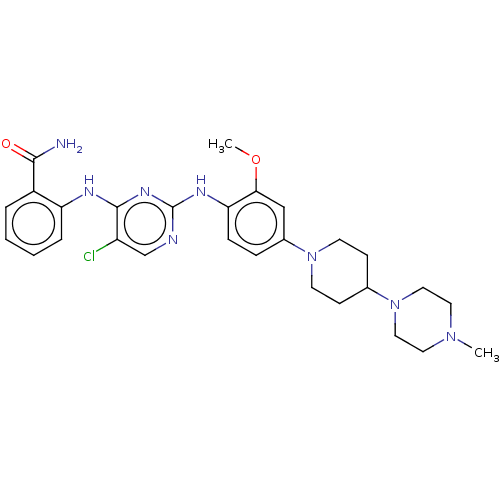
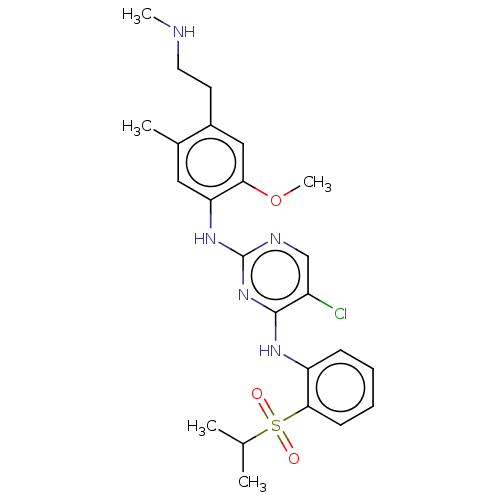
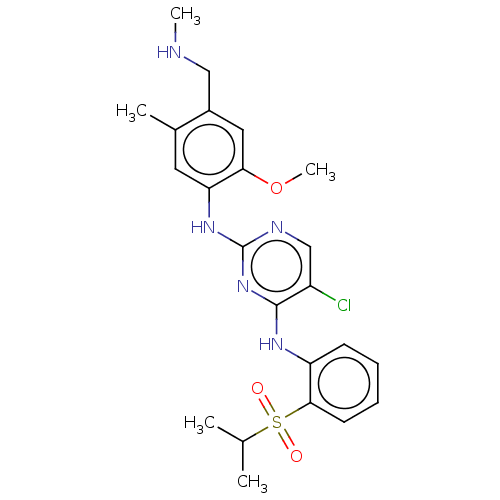
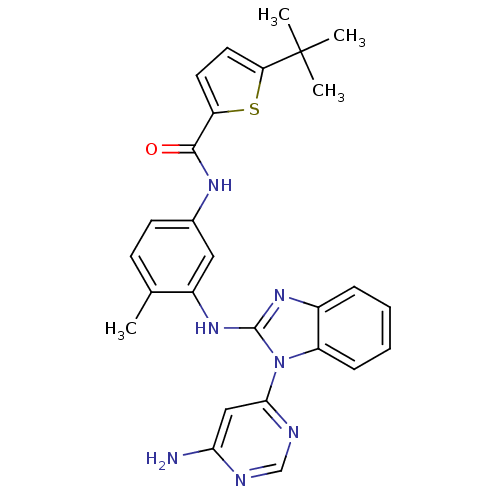
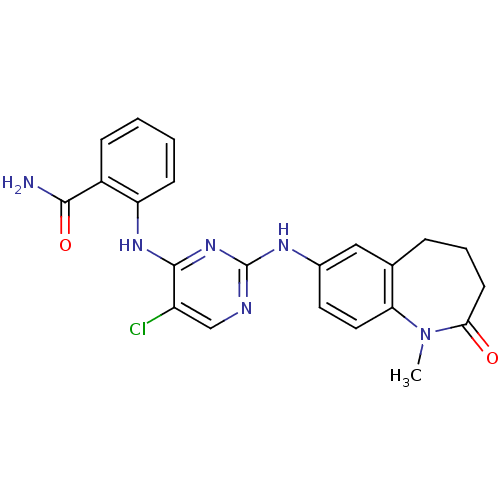
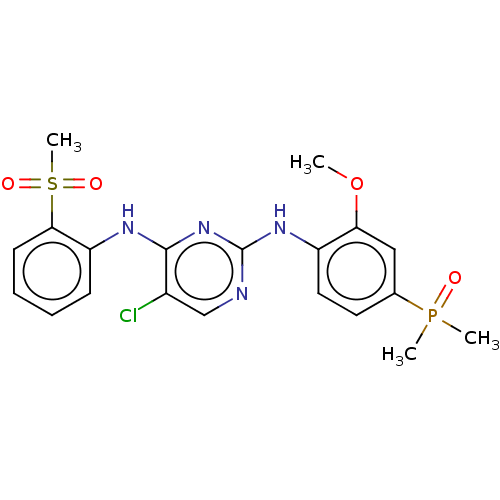
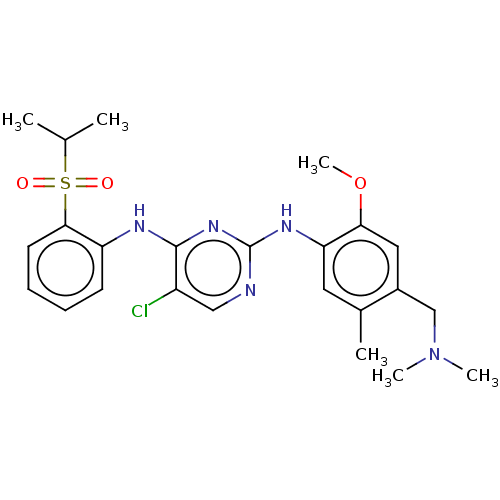
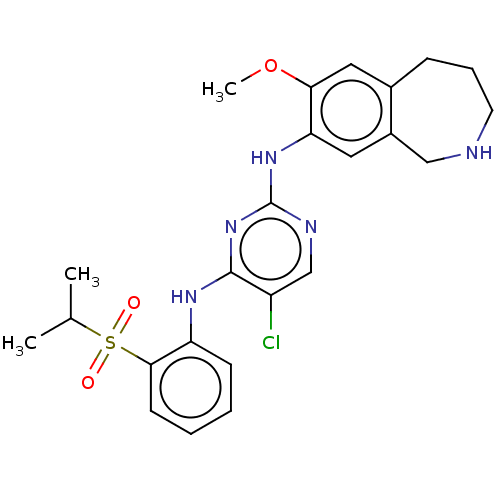
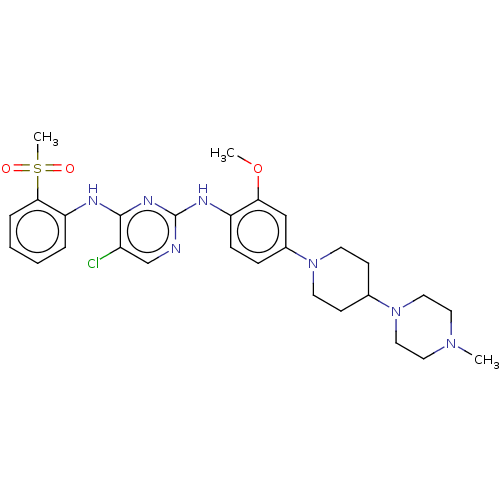
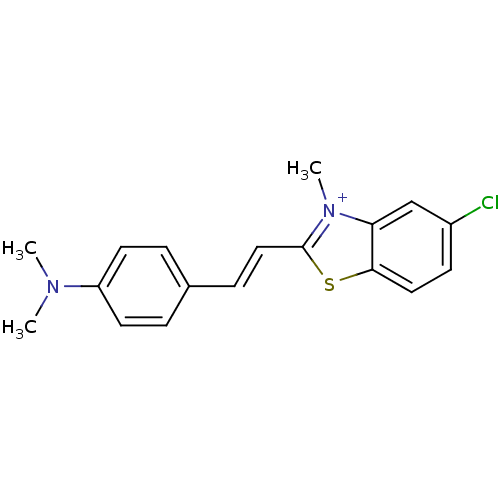
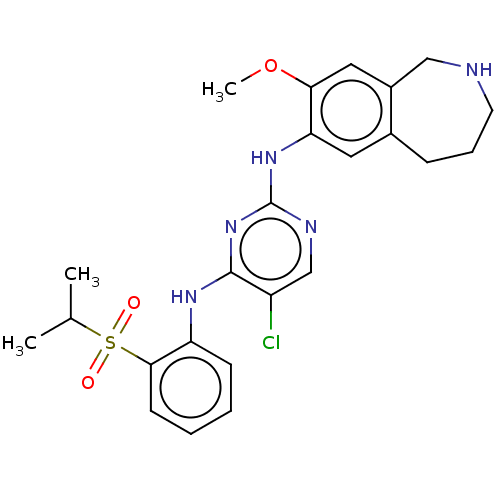
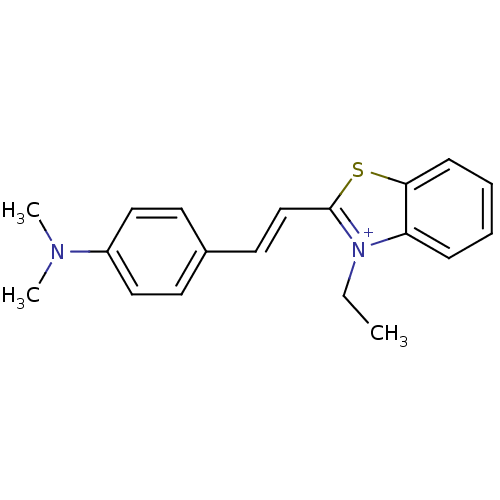
Affinity DataKi: 1.30nMAssay Description:Binding affinity to insulin receptor by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Binding affinity to insulin receptor by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of INSR (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of INSR (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.60E+3nMAssay Description:Inhibition of insulin receptorMore data for this Ligand-Target Pair
Affinity DataKi: >5.24E+3nMAssay Description:Inhibition of INSRMore data for this Ligand-Target Pair
Affinity DataKi: 5.30E+3nMAssay Description:Inhibitory activity against IRKMore data for this Ligand-Target Pair
Affinity DataKi: 8.80E+3nMAssay Description:Inhibitory activity against IRKMore data for this Ligand-Target Pair
Affinity DataKi: 9.00E+3nMAssay Description:Inhibitory activity against IRKMore data for this Ligand-Target Pair
Affinity DataKi: 9.50E+4nMAssay Description:Non-competitive inhibition of human placental insulin receptor expressed in CHO cell membrane assessed as decrease in insulin-stimulated A2 phosphory...More data for this Ligand-Target Pair
Affinity DataIC50: 0.880nMAssay Description:Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Inhibition of human InsR expressed in mouse IGF1R knockout MEF cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Inhibition of IR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of insulin receptor (unknown origin) by homogeneous time resolved fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:In vitro ability to inhibit the binding of [3H]spiperone to dopamine receptor D2 in rat striatal membranes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:In vitro ability to inhibit the binding of [3H]spiperone to dopamine receptor D2 in rat striatal membranes.More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:Inhibition of insulin receptor kinase (unknown origin) using TK as substrate after 30 mins by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Displacement of thioflavin T from insulin receptor by thioflavin-T fluorescent dye assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 3.90nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of Tel-fused InsR expressed in mouse BAF3 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of IR kinaseChecked by AuthorMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of GST-tagged insulin receptor expressed in baculovirus by time-resolved fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.10nMAssay Description:Inhibition of insulin receptor (unknown origin) by homogeneous time resolved fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.10nMAssay Description:Inhibition of human InsR using myelin basic protein as substrate and [gamma-33P]ATP measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataIC50: 5.20nMAssay Description:Displacement of thioflavin T from insulin receptor by thioflavin-T fluorescent dye assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.20nMAssay Description:Inhibition of insulin receptor (unknown origin) by homogeneous time resolved fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:To measure the activity of the N2-(2-methoxyphenyl)pyrimidine derivative represented by formula 1 of the present invention to inhibit anaplastic lymp...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Displacement of thioflavin T from insulin receptor by thioflavin-T fluorescent dye assayMore data for this Ligand-Target Pair