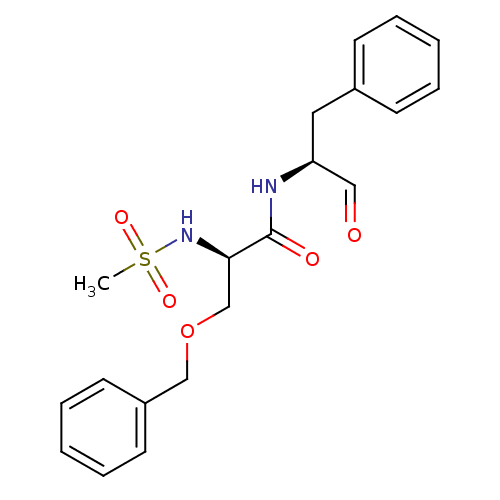
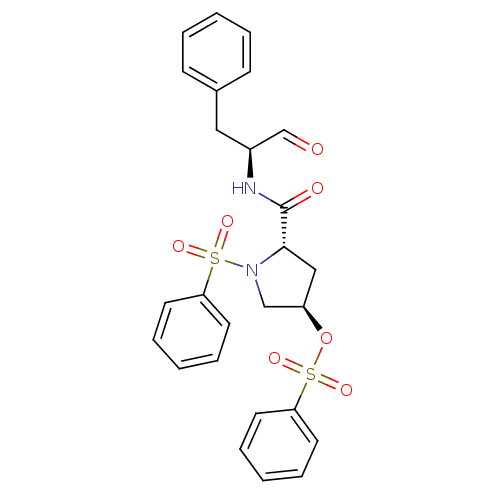
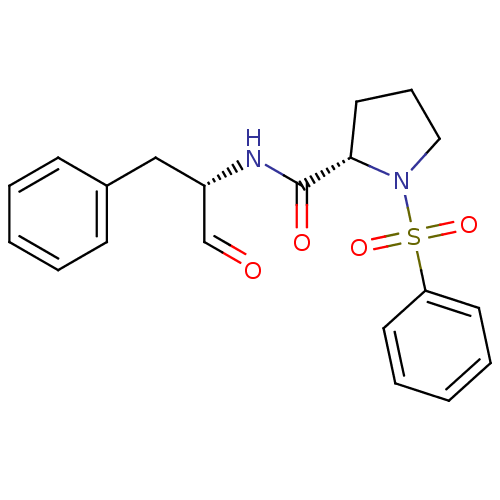
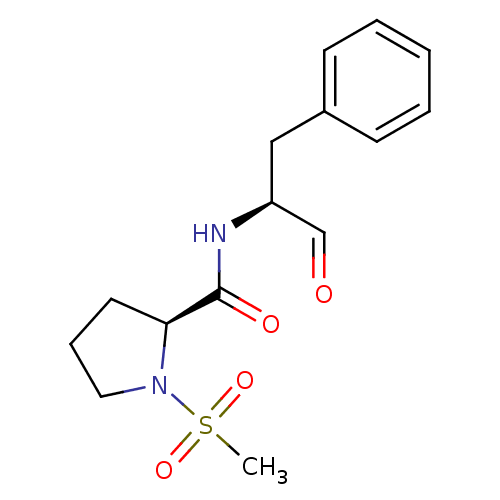
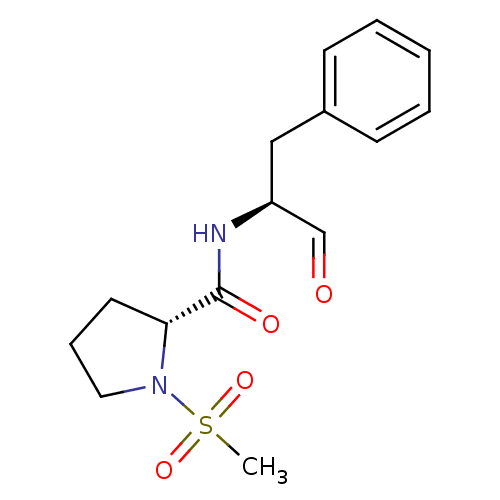
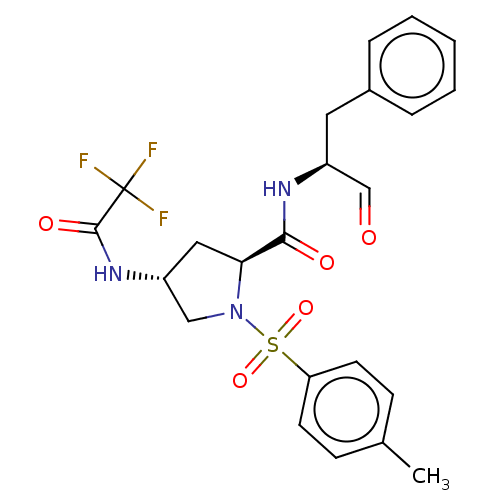
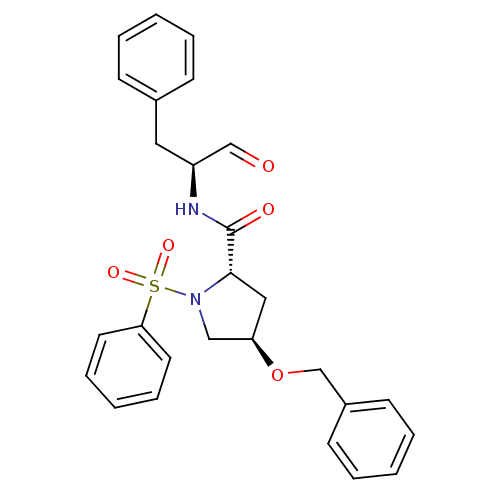
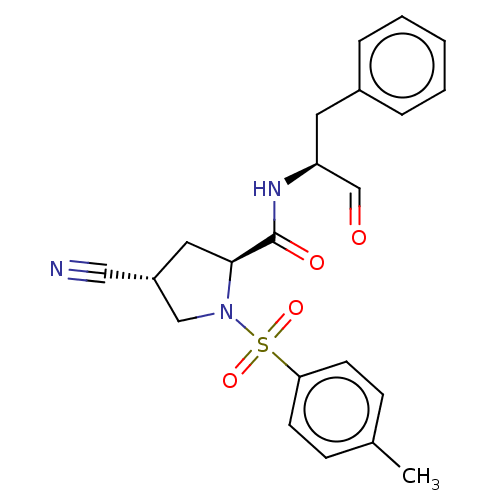
Affinity DataIC50: 11nMAssay Description:Compound was tested for inhibitory activity against recombinant human Calpain 1More data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Compound was tested for inhibitory activity against recombinant human Calpain 1More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Compound was tested for inhibitory activity against recombinant human Calpain 1More data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Compound was tested for inhibitory activity against recombinant human Calpain 1More data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Compound was tested for inhibitory activity against Cathepsin B.More data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Compound was tested for inhibitory activity against recombinant human Calpain 1More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Compound was tested for inhibitory activity against Cathepsin B.More data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Compound was tested for inhibitory activity against recombinant human Calpain 1More data for this Ligand-Target Pair
Affinity DataIC50: 53nMAssay Description:Compound was tested for inhibitory activity against recombinant human Calpain 1More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Compound was tested for inhibitory activity against recombinant human Calpain 1More data for this Ligand-Target Pair
Affinity DataIC50: 125nMAssay Description:Compound was tested for inhibitory activity against recombinant human Calpain 1More data for this Ligand-Target Pair
Affinity DataIC50: 883nMAssay Description:Compound was tested for inhibitory activity against Cathepsin B.More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Compound was tested for inhibitory activity against Cathepsin B.More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Compound was tested for inhibitory activity against Cathepsin B.More data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Compound was tested for inhibitory activity against Cathepsin B.More data for this Ligand-Target Pair
Affinity DataIC50: 8.70E+3nMAssay Description:Compound was tested for inhibitory activity against Cathepsin B.More data for this Ligand-Target Pair
Affinity DataIC50: 8.70E+3nMAssay Description:Compound was tested for inhibitory activity against recombinant human Calpain 1More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Compound was tested for inhibitory activity against Cathepsin B.More data for this Ligand-Target Pair