Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50164240
Substrate
n/a
Meas. Tech.
ChEMBL_304712 (CHEMBL827153)
IC50
800±n/a nM
Citation
 de Dios, A; Shih, C; López de Uralde, B; Sánchez, C; del Prado, M; Martín Cabrejas, LM; Pleite, S; Blanco-Urgoiti, J; Lorite, MJ; Nevill, CR; Bonjouklian, R; York, J; Vieth, M; Wang, Y; Magnus, N; Campbell, RM; Anderson, BD; McCann, DJ; Giera, DD; Lee, PA; Schultz, RM; Li, LC; Johnson, LM; Wolos, JA Design of potent and selective 2-aminobenzimidazole-based p38alpha MAP kinase inhibitors with excellent in vivo efficacy. J Med Chem 48:2270-3 (2005) [PubMed] Article
de Dios, A; Shih, C; López de Uralde, B; Sánchez, C; del Prado, M; Martín Cabrejas, LM; Pleite, S; Blanco-Urgoiti, J; Lorite, MJ; Nevill, CR; Bonjouklian, R; York, J; Vieth, M; Wang, Y; Magnus, N; Campbell, RM; Anderson, BD; McCann, DJ; Giera, DD; Lee, PA; Schultz, RM; Li, LC; Johnson, LM; Wolos, JA Design of potent and selective 2-aminobenzimidazole-based p38alpha MAP kinase inhibitors with excellent in vivo efficacy. J Med Chem 48:2270-3 (2005) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
Inhibitor
Name:
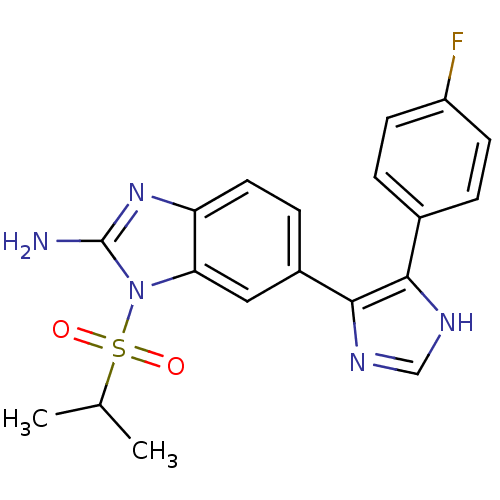
BDBM50164240
Synonyms:
6-[5-(4-Fluoro-phenyl)-3H-imidazol-4-yl]-1-(propane-2-sulfonyl)-1H-benzoimidazol-2-ylamine; Bismesylate salt | CHEMBL192850
Type:
Small organic molecule
Emp. Form.:
C19H18FN5O2S
Mol. Mass.:
399.442
SMILES:
CC(C)S(=O)(=O)n1c(N)nc2ccc(cc12)-c1nc[nH]c1-c1ccc(F)cc1