Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 4F2
Ligand
BDBM558343
Substrate
n/a
Meas. Tech.
Inhibition Test for Each Compound of the Present Invention Against 20-HETE Producing Enzymes (CYP4F2 and CYP4A11)
IC50
28.0±n/a nM
Citation
 Tanaka, H; Kawamura, M; Hamada, M; Kobashi, Y; Ito, Y; Suzuki, K; Bohno, A; Funayama, K Pyridine compound substituted with azole US Patent US11365192 Publication Date 6/21/2022
Tanaka, H; Kawamura, M; Hamada, M; Kobashi, Y; Ito, Y; Suzuki, K; Bohno, A; Funayama, K Pyridine compound substituted with azole US Patent US11365192 Publication Date 6/21/2022 More Info.:
Target
Name:
Cytochrome P450 4F2
Synonyms:
20-HETE synthase | 20-hydroxyeicosatetraenoic acid synthase | Arachidonic acid omega-hydroxylase | CP4F2_HUMAN | CYP4F2 | CYPIVF2 | Cytochrome P450 4F2 | Cytochrome P450-LTB-omega | Leukotriene-B(4) 20-monooxygenase 1 | Leukotriene-B(4) omega-hydroxylase 1 | Phylloquinone omega-hydroxylase CYP4F2
Type:
PROTEIN
Mol. Mass.:
59859.32
Organism:
Homo sapiens (Human)
Description:
ChEMBL_10743
Residue:
520
Sequence:
MSQLSLSWLGLWPVAASPWLLLLLVGASWLLAHVLAWTYAFYDNCRRLRCFPQPPRRNWFWGHQGMVNPTEEGMRVLTQLVATYPQGFKVWMGPISPLLSLCHPDIIRSVINASAAIAPKDKFFYSFLEPWLGDGLLLSAGDKWSRHRRMLTPAFHFNILKPYMKIFNESVNIMHAKWQLLASEGSACLDMFEHISLMTLDSLQKCVFSFDSHCQEKPSEYIAAILELSALVSKRHHEILLHIDFLYYLTPDGQRFRRACRLVHDFTDAVIQERRRTLPSQGVDDFLQAKAKSKTLDFIDVLLLSKDEDGKKLSDEDIRAEADTFMFEGHDTTASGLSWVLYHLAKHPEYQERCRQEVQELLKDREPKEIEWDDLAHLPFLTMCMKESLRLHPPVPVISRHVTQDIVLPDGRVIPKGIICLISVFGTHHNPAVWPDPEVYDPFRFDPENIKERSPLAFIPFSAGPRNCIGQTFAMAEMKVVLALTLLRFRVLPDHTEPRRKPELVLRAEGGLWLRVEPLS
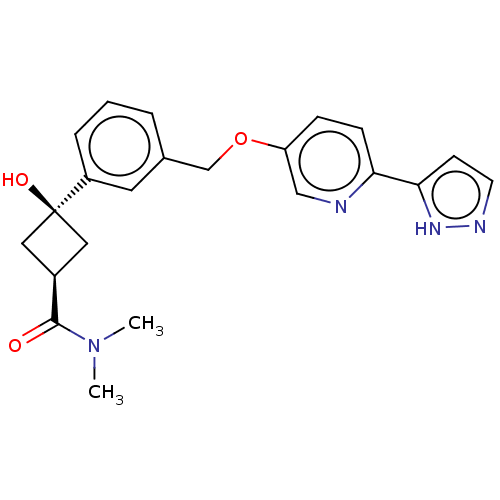
Inhibitor
Name:
BDBM558343
Synonyms:
US11365192, Example 42-21
Type:
Small organic molecule
Emp. Form.:
C22H24N4O3
Mol. Mass.:
392.451
SMILES:
CN(C)C(=O)[C@H]1C[C@@](O)(C1)c1cccc(COc2ccc(nc2)-c2ccn[nH]2)c1 |r,wD:7.7,5.4,(-8.83,.07,;-8.83,-1.47,;-10.16,-2.24,;-7.5,-2.24,;-7.5,-3.78,;-6.16,-1.47,;-4.68,-1.87,;-4.28,-.38,;-4.28,1.16,;-5.76,.02,;-2.94,-1.15,;-2.94,-2.69,;-1.61,-3.46,;-.28,-2.69,;-.28,-1.15,;1.06,-.38,;2.39,-1.15,;3.73,-.38,;5.06,-1.15,;6.39,-.38,;6.39,1.16,;5.06,1.93,;3.73,1.16,;7.73,1.93,;9.13,1.3,;10.16,2.45,;9.39,3.78,;7.89,3.46,;-1.61,-.38,)|