Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Coagulation factor X
Ligand
BDBM14144
Substrate
BDBM12989
Meas. Tech.
Enzyme Assay and Determination of the Inhibition Constants
Ki
1400±n/a nM
Citation
 Katz, BA; Sprengeler, PA; Luong, C; Verner, E; Elrod, K; Kirtley, M; Janc, J; Spencer, JR; Breitenbucher, JG; Hui, H; McGee, D; Allen, D; Martelli, A; Mackman, RL Engineering inhibitors highly selective for the S1 sites of Ser190 trypsin-like serine protease drug targets. Chem Biol 8:1107-21 (2001) [PubMed] Article
Katz, BA; Sprengeler, PA; Luong, C; Verner, E; Elrod, K; Kirtley, M; Janc, J; Spencer, JR; Breitenbucher, JG; Hui, H; McGee, D; Allen, D; Martelli, A; Mackman, RL Engineering inhibitors highly selective for the S1 sites of Ser190 trypsin-like serine protease drug targets. Chem Biol 8:1107-21 (2001) [PubMed] Article More Info.:
Target
Name:
Coagulation factor X
Synonyms:
Activated coagulation factor X (FXa) | Activated factor Xa heavy chain | Coagulation factor X precursor | Coagulation factor Xa | F10 | FA10_HUMAN | Factor X heavy chain | Factor X light chain | Factor Xa | Stuart factor | Stuart-Prower factor
Type:
Enzyme
Mol. Mass.:
54726.60
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
488
Sequence:
MGRPLHLVLLSASLAGLLLLGESLFIRREQANNILARVTRANSFLEEMKKGHLERECMEETCSYEEAREVFEDSDKTNEFWNKYKDGDQCETSPCQNQGKCKDGLGEYTCTCLEGFEGKNCELFTRKLCSLDNGDCDQFCHEEQNSVVCSCARGYTLADNGKACIPTGPYPCGKQTLERRKRSVAQATSSSGEAPDSITWKPYDAADLDPTENPFDLLDFNQTQPERGDNNLTRIVGGQECKDGECPWQALLINEENEGFCGGTILSEFYILTAAHCLYQAKRFKVRVGDRNTEQEEGGEAVHEVEVVIKHNRFTKETYDFDIAVLRLKTPITFRMNVAPACLPERDWAESTLMTQKTGIVSGFGRTHEKGRQSTRLKMLEVPYVDRNSCKLSSSFIITQNMFCAGYDTKQEDACQGDSGGPHVTRFKDTYFVTGIVSWGEGCARKGKYGIYTKVTAFLKWIDRSMKTRGLPKAKSHAPEVITSSPLK
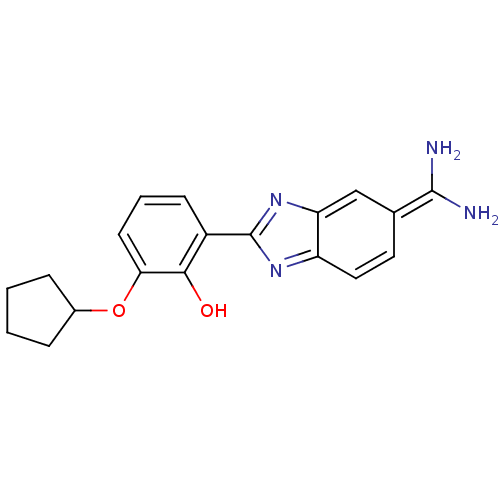
Inhibitor
Name:
BDBM14144
Synonyms:
2-{5-[amino(iminiumyl)methyl]-1H-1,3-benzodiazol-2-yl}-6-(cyclopentyloxy)benzen-1-olate | APC-10655 | CA-06
Type:
Small organic molecule
Emp. Form.:
C19H20N4O2
Mol. Mass.:
336.3877
SMILES:
NC(=[NH2+])c1ccc2nc([nH]c2c1)-c1cccc(OC2CCCC2)c1[O-]
Substrate
Name:
BDBM12989
Synonyms:
CH3OCO-D-CHA-Gly-Arg-pNA.AcOH | Chromogenic Substrate Pefachrome FXa | acetic acid; methyl N-[(1R)-1-[({[(1S)-4-carbamimidamido-1-[(4-nitrophenyl)carbamoyl]butyl]carbamoyl}methyl)carbamoyl]-2-cyclohexylethyl]carbamate
Type:
Small organic molecule
Emp. Form.:
C25H38N8O7
Mol. Mass.:
562.6186
SMILES:
COC(=O)N[C@H](CC1CCCCC1)C(=O)NCC(=O)N[C@@H](CCC[NH+]=C(N)[NH-])C(=O)Nc1ccc(cc1)[N+]([O-])=O |r,w:24.24|