Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Plasminogen
Ligand
BDBM23895
Substrate
BDBM23923
Meas. Tech.
Determination of Inhibition Constants
Ki
3100±n/a nM
Citation
 Steinmetzer, T; Schweinitz, A; Stürzebecher, A; Dönnecke, D; Uhland, K; Schuster, O; Steinmetzer, P; Müller, F; Friedrich, R; Than, ME; Bode, W; Stürzebecher, J Secondary amides of sulfonylated 3-amidinophenylalanine. New potent and selective inhibitors of matriptase. J Med Chem 49:4116-26 (2006) [PubMed] Article
Steinmetzer, T; Schweinitz, A; Stürzebecher, A; Dönnecke, D; Uhland, K; Schuster, O; Steinmetzer, P; Müller, F; Friedrich, R; Than, ME; Bode, W; Stürzebecher, J Secondary amides of sulfonylated 3-amidinophenylalanine. New potent and selective inhibitors of matriptase. J Med Chem 49:4116-26 (2006) [PubMed] Article More Info.:
Target
Name:
Plasminogen
Synonyms:
Activation peptide | Angiostatin | PLG | PLMN_HUMAN | Plasmin | Plasmin heavy chain A | Plasmin heavy chain A, short form | Plasmin light chain B
Type:
Enzyme
Mol. Mass.:
90579.18
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
810
Sequence:
MEHKEVVLLLLLFLKSGQGEPLDDYVNTQGASLFSVTKKQLGAGSIEECAAKCEEDEEFTCRAFQYHSKEQQCVIMAENRKSSIIIRMRDVVLFEKKVYLSECKTGNGKNYRGTMSKTKNGITCQKWSSTSPHRPRFSPATHPSEGLEENYCRNPDNDPQGPWCYTTDPEKRYDYCDILECEEECMHCSGENYDGKISKTMSGLECQAWDSQSPHAHGYIPSKFPNKNLKKNYCRNPDRELRPWCFTTDPNKRWELCDIPRCTTPPPSSGPTYQCLKGTGENYRGNVAVTVSGHTCQHWSAQTPHTHNRTPENFPCKNLDENYCRNPDGKRAPWCHTTNSQVRWEYCKIPSCDSSPVSTEQLAPTAPPELTPVVQDCYHGDGQSYRGTSSTTTTGKKCQSWSSMTPHRHQKTPENYPNAGLTMNYCRNPDADKGPWCFTTDPSVRWEYCNLKKCSGTEASVVAPPPVVLLPDVETPSEEDCMFGNGKGYRGKRATTVTGTPCQDWAAQEPHRHSIFTPETNPRAGLEKNYCRNPDGDVGGPWCYTTNPRKLYDYCDVPQCAAPSFDCGKPQVEPKKCPGRVVGGCVAHPHSWPWQVSLRTRFGMHFCGGTLISPEWVLTAAHCLEKSPRPSSYKVILGAHQEVNLEPHVQEIEVSRLFLEPTRKDIALLKLSSPAVITDKVIPACLPSPNYVVADRTECFITGWGETQGTFGAGLLKEAQLPVIENKVCNRYEFLNGRVQSTELCAGHLAGGTDSCQGDSGGPLVCFEKDKYILQGVTSWGLGCARPNKPGVYVRVSRFVTWIEGVMRNN
Inhibitor
Name:
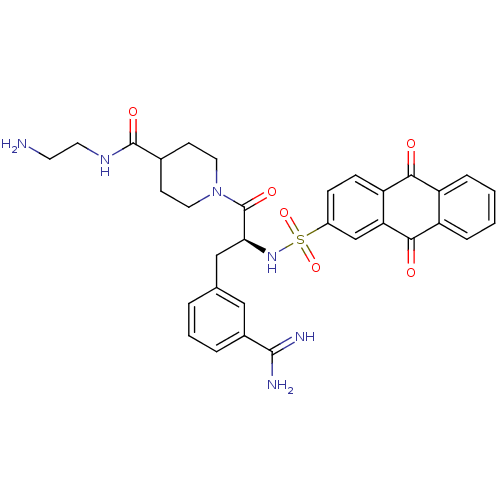
BDBM23895
Synonyms:
3-amidinophenylalanine deriv., 39 | N-(2-aminoethyl)-1-[(2S)-3-(3-carbamimidoylphenyl)-2-[(9,10-dioxo-9,10-dihydroanthracene-2-)sulfonamido]propanoyl]piperidine-4-carboxamide
Type:
Small organic molecule
Emp. Form.:
C32H34N6O6S
Mol. Mass.:
630.714
SMILES:
NCCNC(=O)C1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1ccc2C(=O)c3ccccc3C(=O)c2c1 |r|