Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
5-hydroxytryptamine receptor 1A
Ligand
BDBM263371
Substrate
n/a
Meas. Tech.
5-HT1A Receptor Binding Test
pH
7.4±n/a
Ki
8.96±n/a nM
Comments
extracted
Citation
More Info.:
Target
Name:
5-hydroxytryptamine receptor 1A
Synonyms:
5-HT-1A | 5-HT1A | 5-hydroxytryptamine receptor 1A (5-HT-1A) | 5HT1A_HUMAN | ADRB2RL1 | ADRBRL1 | Dopamine D2 receptor and serotonin 1a receptor | G-21 | HTR1A | Serotonin receptor 1A
Type:
n/a
Mol. Mass.:
46122.49
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
422
Sequence:
MDVLSPGQGNNTTSPPAPFETGGNTTGISDVTVSYQVITSLLLGTLIFCAVLGNACVVAAIALERSLQNVANYLIGSLAVTDLMVSVLVLPMAALYQVLNKWTLGQVTCDLFIALDVLCCTSSILHLCAIALDRYWAITDPIDYVNKRTPRRAAALISLTWLIGFLISIPPMLGWRTPEDRSDPDACTISKDHGYTIYSTFGAFYIPLLLMLVLYGRIFRAARFRIRKTVKKVEKTGADTRHGASPAPQPKKSVNGESGSRNWRLGVESKAGGALCANGAVRQGDDGAALEVIEVHRVGNSKEHLPLPSEAGPTPCAPASFERKNERNAEAKRKMALARERKTVKTLGIIMGTFILCWLPFFIVALVLPFCESSCHMPTLLGAIINWLGYSNSLLNPVIYAYFNKDFQNAFKKIIKCKFCRQ
Inhibitor
Name:
BDBM263371
Synonyms:
US9550741, I-5
Type:
Small organic molecule
Emp. Form.:
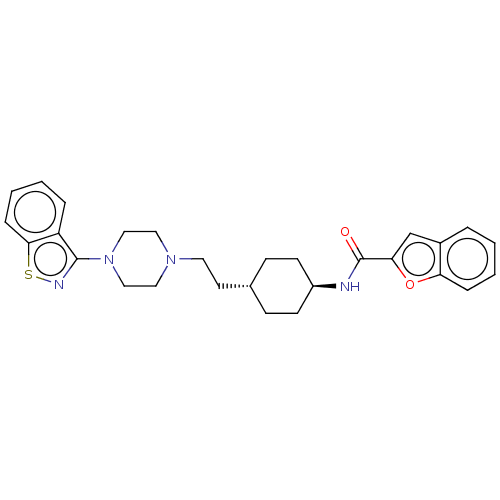
C28H32N4O2S
Mol. Mass.:
488.644
SMILES:
O=C(N[C@H]1CC[C@H](CCN2CCN(CC2)c2nsc3ccccc23)CC1)c1cc2ccccc2o1 |r,wU:3.2,wD:6.6,(-5.89,-.2,;-5.89,1.34,;-4.56,2.11,;-3.22,1.34,;-1.89,2.11,;-.56,1.34,;-.56,-.2,;.78,-.97,;2.11,-.2,;3.44,-.97,;3.44,-2.51,;4.78,-3.28,;6.11,-2.51,;6.11,-.97,;4.78,-.2,;7.45,-3.28,;7.45,-4.82,;8.91,-5.3,;9.81,-4.05,;11.35,-3.89,;11.97,-2.48,;11.07,-1.24,;9.54,-1.4,;8.91,-2.8,;-1.89,-.97,;-3.22,-.2,;-7.23,2.11,;-7.39,3.64,;-8.89,3.96,;-9.66,5.3,;-11.2,5.3,;-11.97,3.96,;-11.2,2.63,;-9.66,2.63,;-8.63,1.48,)|