Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Apoptotic protease-activating factor 1
Ligand
BDBM83973
Substrate
n/a
Meas. Tech.
Dose response confirmation of uHTS hits for Apaf-1 in a Fluorescent assay
IC50
443±n/a nM
Citation
 PubChem, PC Dose response confirmation of uHTS hits for Apaf-1 in a Fluorescent assay PubChem Bioassay (2011)[AID]
PubChem, PC Dose response confirmation of uHTS hits for Apaf-1 in a Fluorescent assay PubChem Bioassay (2011)[AID] More Info.:
Target
Name:
Apoptotic protease-activating factor 1
Synonyms:
APAF1 | APAF_HUMAN | Apoptotic peptidase activating factor 1 | KIAA0413
Type:
Enzyme Catalytic Domain
Mol. Mass.:
141834.40
Organism:
Homo sapiens (Human)
Description:
O14727
Residue:
1248
Sequence:
MDAKARNCLLQHREALEKDIKTSYIMDHMISDGFLTISEEEKVRNEPTQQQRAAMLIKMILKKDNDSYVSFYNALLHEGYKDLAALLHDGIPVVSSSSGKDSVSGITSYVRTVLCEGGVPQRPVVFVTRKKLVNAIQQKLSKLKGEPGWVTIHGMAGCGKSVLAAEAVRDHSLLEGCFPGGVHWVSVGKQDKSGLLMKLQNLCTRLDQDESFSQRLPLNIEEAKDRLRILMLRKHPRSLLILDDVWDSWVLKAFDSQCQILLTTRDKSVTDSVMGPKYVVPVESSLGKEKGLEILSLFVNMKKADLPEQAHSIIKECKGSPLVVSLIGALLRDFPNRWEYYLKQLQNKQFKRIRKSSSYDYEALDEAMSISVEMLREDIKDYYTDLSILQKDVKVPTKVLCILWDMETEEVEDILQEFVNKSLLFCDRNGKSFRYYLHDLQVDFLTEKNCSQLQDLHKKIITQFQRYHQPHTLSPDQEDCMYWYNFLAYHMASAKMHKELCALMFSLDWIKAKTELVGPAHLIHEFVEYRHILDEKDCAVSENFQEFLSLNGHLLGRQPFPNIVQLGLCEPETSEVYQQAKLQAKQEVDNGMLYLEWINKKNITNLSRLVVRPHTDAVYHACFSEDGQRIASCGADKTLQVFKAETGEKLLEIKAHEDEVLCCAFSTDDRFIATCSVDKKVKIWNSMTGELVHTYDEHSEQVNCCHFTNSSHHLLLATGSSDCFLKLWDLNQKECRNTMFGHTNSVNHCRFSPDDKLLASCSADGTLKLWDATSANERKSINVKQFFLNLEDPQEDMEVIVKCCSWSADGARIMVAAKNKIFLFDIHTSGLLGEIHTGHHSTIQYCDFSPQNHLAVVALSQYCVELWNTDSRSKVADCRGHLSWVHGVMFSPDGSSFLTSSDDQTIRLWETKKVCKNSAVMLKQEVDVVFQENEVMVLAVDHIRRLQLINGRTGQIDYLTEAQVSCCCLSPHLQYIAFGDENGAIEILELVNNRIFQSRFQHKKTVWHIQFTADEKTLISSSDDAEIQVWNWQLDKCIFLRGHQETVKDFRLLKNSRLLSWSFDGTVKVWNIITGNKEKDFVCHQGTVLSCDISHDATKFSSTSADKTAKIWSFDLLLPLHELRGHNGCVRCSAFSVDSTLLATGDDNGEIRIWNVSNGELLHLCAPLSEEGAATHGGWVTDLCFSPDGKMLISAGGYIKWWNVVTGESSQTFYTNGTNLKKIHVSPDFKTYVTVDNLGILYILQTLE
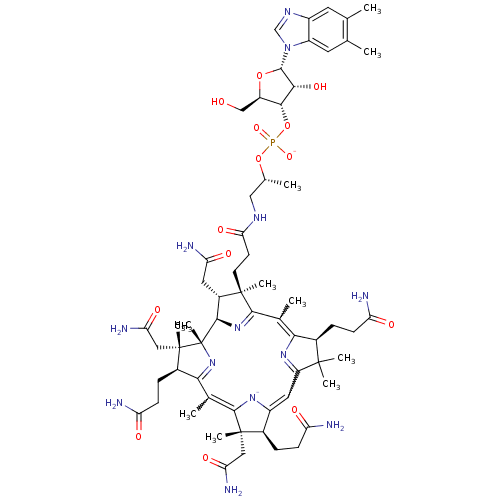
Inhibitor
Name:
BDBM83973
Synonyms:
CYANOCOBALAMIN | Cobalamin | MLS002153809 | RUVITE | SMR001233181 | cid_25102581 | vitamin B12
Type:
Small organic molecule
Emp. Form.:
C62H88N13O14P
Mol. Mass.:
1270.4157
SMILES:
C[C@H](CNC(=O)CC[C@]1(C)[C@@H](CC(N)=O)[C@H]2N=C1C(C)=C1N=C(C=C3[N-]C(=C(C)C4=N[C@]2(C)[C@@](C)(CC(N)=O)[C@@H]4CCC(N)=O)[C@@](C)(CC(N)=O)[C@@H]3CCC(N)=O)C(C)(C)[C@@H]1CCC(N)=O)OP([O-])(=O)O[C@@H]1[C@@H](CO)O[C@@H]([C@@H]1O)n1cnc2cc(C)c(C)cc12 |w:27.28,18.19,23.23,c:16,t:22,30|