Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glutamate carboxypeptidase 2
Ligand
BDBM50454862
Substrate
n/a
Meas. Tech.
ChEMBL_1753489 (CHEMBL4188249)
Ki
0.140000±n/a nM
Citation
More Info.:
Target
Name:
Glutamate carboxypeptidase 2
Synonyms:
FGCP | FOLH | FOLH1 | FOLH1_HUMAN | Folate hydrolase 1 | Folylpoly-gamma-glutamate carboxypeptidase | Glutamate carboxypeptidase 2 | Glutamate carboxypeptidase II | Membrane glutamate carboxypeptidase | N-acetylated-alpha-linked acidic dipeptidase I | NAALAD1 | NAALADase I | PSM | PSMA | Prostate-specific membrane antigen | Pteroylpoly-gamma-glutamate carboxypeptidase | mGCP
Type:
PROTEIN
Mol. Mass.:
84333.66
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1497035
Residue:
750
Sequence:
MWNLLHETDSAVATARRPRWLCAGALVLAGGFFLLGFLFGWFIKSSNEATNITPKHNMKAFLDELKAENIKKFLYNFTQIPHLAGTEQNFQLAKQIQSQWKEFGLDSVELAHYDVLLSYPNKTHPNYISIINEDGNEIFNTSLFEPPPPGYENVSDIVPPFSAFSPQGMPEGDLVYVNYARTEDFFKLERDMKINCSGKIVIARYGKVFRGNKVKNAQLAGAKGVILYSDPADYFAPGVKSYPDGWNLPGGGVQRGNILNLNGAGDPLTPGYPANEYAYRRGIAEAVGLPSIPVHPIGYYDAQKLLEKMGGSAPPDSSWRGSLKVPYNVGPGFTGNFSTQKVKMHIHSTNEVTRIYNVIGTLRGAVEPDRYVILGGHRDSWVFGGIDPQSGAAVVHEIVRSFGTLKKEGWRPRRTILFASWDAEEFGLLGSTEWAEENSRLLQERGVAYINADSSIEGNYTLRVDCTPLMYSLVHNLTKELKSPDEGFEGKSLYESWTKKSPSPEFSGMPRISKLGSGNDFEVFFQRLGIASGRARYTKNWETNKFSGYPLYHSVYETYELVEKFYDPMFKYHLTVAQVRGGMVFELANSIVLPFDCRDYAVVLRKYADKIYSISMKHPQEMKTYSVSFDSLFSAVKNFTEIASKFSERLQDFDKSNPIVLRMMNDQLMFLERAFIDPLGLPDRPFYRHVIYAPSSHNKYAGESFPGIYDALFDIESKVDPSKAWGEVKRQIYVAAFTVQAAAETLSEVA
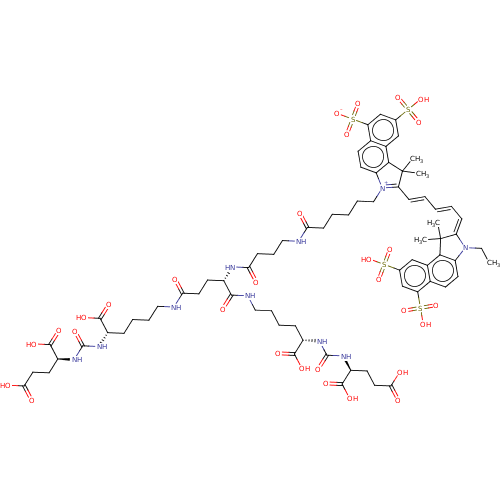
Inhibitor
Name:
BDBM50454862
Synonyms:
CHEMBL4211875
Type:
Small organic molecule
Emp. Form.:
C74H96N10O30S4
Mol. Mass.:
1733.863
SMILES:
CCN1\C(=C\C=C\C=C\C2=[N+](CCCCCC(=O)NCCCC(=O)N[C@@H](CCC(=O)NCCCC[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)C(=O)NCCCC[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O)c3ccc4c(cc(cc4c3C2(C)C)S(O)(=O)=O)S([O-])(=O)=O)C(C)(C)c2c1ccc1c(cc(cc21)S(O)(=O)=O)S(O)(=O)=O |r,c:9|