Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
Ligand
BDBM50092783
Substrate
n/a
Meas. Tech.
ChEMBL_1804141 (CHEMBL4276433)
Kd
7100±n/a nM
Citation
 Rageot, D; Bohnacker, T; Melone, A; Langlois, JB; Borsari, C; Hillmann, P; Sele, AM; Beaufils, F; Zvelebil, M; Hebeisen, P; Löscher, W; Burke, J; Fabbro, D; Wymann, MP Discovery and Preclinical Characterization of 5-[4,6-Bis({3-oxa-8-azabicyclo[3.2.1]octan-8-yl})-1,3,5-triazin-2-yl]-4-(difluoromethyl)pyridin-2-amine (PQR620), a Highly Potent and Selective mTORC1/2 Inhibitor for Cancer and Neurological Disorders. J Med Chem 61:10084-10105 (2018) [PubMed] Article
Rageot, D; Bohnacker, T; Melone, A; Langlois, JB; Borsari, C; Hillmann, P; Sele, AM; Beaufils, F; Zvelebil, M; Hebeisen, P; Löscher, W; Burke, J; Fabbro, D; Wymann, MP Discovery and Preclinical Characterization of 5-[4,6-Bis({3-oxa-8-azabicyclo[3.2.1]octan-8-yl})-1,3,5-triazin-2-yl]-4-(difluoromethyl)pyridin-2-amine (PQR620), a Highly Potent and Selective mTORC1/2 Inhibitor for Cancer and Neurological Disorders. J Med Chem 61:10084-10105 (2018) [PubMed] Article More Info.:
Target
Name:
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
Synonyms:
PI3-kinase p110 subunit gamma | PI3-kinase subunit p120-gamma | PI3Kgamma | PIK3CG | PK3CG_HUMAN | Phosphatidylinositol 4,5-biphosphate 3-kinase catalytic subunit gamma (PIK3CG) | Phosphatidylinositol 4,5-bisphosphate 3-kinase (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase 110 kDa catalytic subunit gamma (PI3K gamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma (PI3Kgamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3K gamma) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (PI3Kgamma) | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma isoform | Phosphoinositide 3-Kinase (PI3K), gamma Chain A | Phosphoinositide 3-kinases gamma (PI3K gamma) | Phosphoinositide-3-kinase (PI3K gamma) | p120-PI3K
Type:
Enzyme Subunit
Mol. Mass.:
126470.30
Organism:
Homo sapiens (Human)
Description:
P48736
Residue:
1102
Sequence:
MELENYKQPVVLREDNCRRRRRMKPRSAAASLSSMELIPIEFVLPTSQRKCKSPETALLHVAGHGNVEQMKAQVWLRALETSVAADFYHRLGPHHFLLLYQKKGQWYEIYDKYQVVQTLDCLRYWKATHRSPGQIHLVQRHPPSEESQAFQRQLTALIGYDVTDVSNVHDDELEFTRRGLVTPRMAEVASRDPKLYAMHPWVTSKPLPEYLWKKIANNCIFIVIHRSTTSQTIKVSPDDTPGAILQSFFTKMAKKKSLMDIPESQSEQDFVLRVCGRDEYLVGETPIKNFQWVRHCLKNGEEIHVVLDTPPDPALDEVRKEEWPLVDDCTGVTGYHEQLTIHGKDHESVFTVSLWDCDRKFRVKIRGIDIPVLPRNTDLTVFVEANIQHGQQVLCQRRTSPKPFTEEVLWNVWLEFSIKIKDLPKGALLNLQIYCGKAPALSSKASAESPSSESKGKVQLLYYVNLLLIDHRFLLRRGEYVLHMWQISGKGEDQGSFNADKLTSATNPDKENSMSISILLDNYCHPIALPKHQPTPDPEGDRVRAEMPNQLRKQLEAIIATDPLNPLTAEDKELLWHFRYESLKHPKAYPKLFSSVKWGQQEIVAKTYQLLARREVWDQSALDVGLTMQLLDCNFSDENVRAIAVQKLESLEDDDVLHYLLQLVQAVKFEPYHDSALARFLLKRGLRNKRIGHFLFWFLRSEIAQSRHYQQRFAVILEAYLRGCGTAMLHDFTQQVQVIEMLQKVTLDIKSLSAEKYDVSSQVISQLKQKLENLQNSQLPESFRVPYDPGLKAGALAIEKCKVMASKKKPLWLEFKCADPTALSNETIGIIFKHGDDLRQDMLILQILRIMESIWETESLDLCLLPYGCISTGDKIGMIEIVKDATTIAKIQQSTVGNTGAFKDEVLNHWLKEKSPTEEKFQAAVERFVYSCAGYCVATFVLGIGDRHNDNIMITETGNLFHIDFGHILGNYKSFLGINKERVPFVLTPDFLFVMGTSGKKTSPHFQKFQDICVKAYLALRHHTNLLIILFSMMLMTGMPQLTSKEDIEYIRDALTVGKNEEDAKKYFLDQIEVCRDKGWTVQFNWFLHLVLGIKQGEKHSA
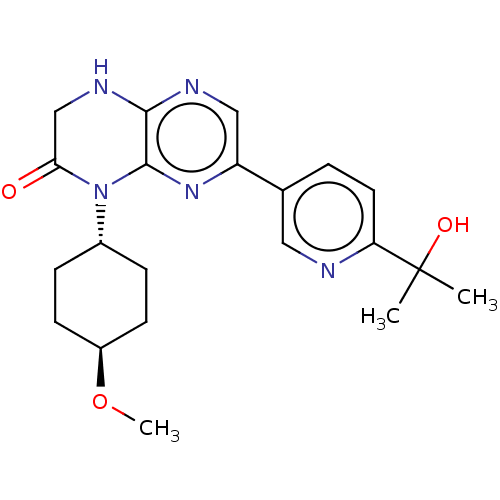
Inhibitor
Name:
BDBM50092783
Synonyms:
CHEMBL3586404
Type:
Small organic molecule
Emp. Form.:
C21H27N5O3
Mol. Mass.:
397.4708
SMILES:
CO[C@H]1CC[C@@H](CC1)N1C(=O)CNc2ncc(nc12)-c1ccc(nc1)C(C)(C)O |r,wU:2.1,wD:5.8,(2.39,-8.32,;1.33,-7.7,;1.33,-6.16,;-.01,-5.39,;-0,-3.85,;1.33,-3.08,;2.66,-3.85,;2.66,-5.39,;1.33,-1.54,;2.66,-.77,;3.73,-1.38,;2.66,.77,;1.33,1.54,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;-4.01,-1.54,;-4.01,-3.08,;-5.35,-3.85,;-6.68,-3.08,;-6.68,-1.54,;-5.35,-.77,;-8.02,-3.85,;-9.08,-3.23,;-9.08,-4.47,;-8.02,-5.08,)|