Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Neuropeptide Y receptor type 4
Ligand
BDBM50532413
Substrate
n/a
Meas. Tech.
ChEMBL_1923152 (CHEMBL4426108)
EC50
3.7±n/a nM
Citation
 Liu, M; Mountford, SJ; Richardson, RR; Groenen, M; Holliday, ND; Thompson, PE Optically Pure, Structural, and Fluorescent Analogues of a Dimeric Y4 Receptor Agonist Derived by an Olefin Metathesis Approach. J Med Chem 59:6059-69 (2016) [PubMed] Article
Liu, M; Mountford, SJ; Richardson, RR; Groenen, M; Holliday, ND; Thompson, PE Optically Pure, Structural, and Fluorescent Analogues of a Dimeric Y4 Receptor Agonist Derived by an Olefin Metathesis Approach. J Med Chem 59:6059-69 (2016) [PubMed] Article More Info.:
Target
Name:
Neuropeptide Y receptor type 4
Synonyms:
NPY-Y4 | NPY4-R | NPY4R | NPY4R_HUMAN | PP1 | PPYR1 | Pancreatic polypeptide receptor 1
Type:
Enzyme Catalytic Domain
Mol. Mass.:
42207.58
Organism:
Homo sapiens (Human)
Description:
NPY-Y4 PPYR1 HUMAN::P50391
Residue:
375
Sequence:
MNTSHLLALLLPKSPQGENRSKPLGTPYNFSEHCQDSVDVMVFIVTSYSIETVVGVLGNLCLMCVTVRQKEKANVTNLLIANLAFSDFLMCLLCQPLTAVYTIMDYWIFGETLCKMSAFIQCMSVTVSILSLVLVALERHQLIINPTGWKPSISQAYLGIVLIWVIACVLSLPFLANSILENVFHKNHSKALEFLADKVVCTESWPLAHHRTIYTTFLLLFQYCLPLGFILVCYARIYRRLQRQGRVFHKGTYSLRAGHMKQVNVVLVVMVVAFAVLWLPLHVFNSLEDWHHEAIPICHGNLIFLVCHLLAMASTCVNPFIYGFLNTNFKKEIKALVLTCQQSAPLEESEHLPLSTVHTEVSKGSLRLSGRSNPI
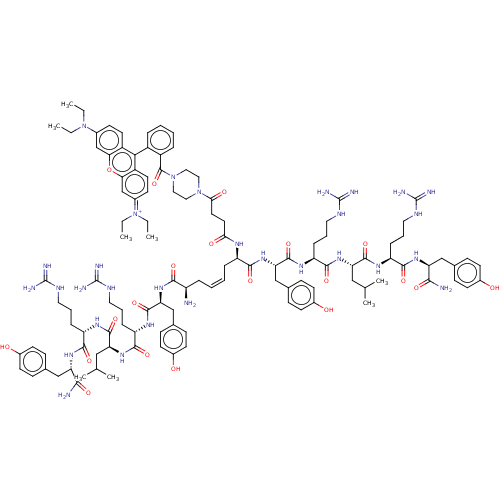
Inhibitor
Name:
BDBM50532413
Synonyms:
CHEMBL4546224
Type:
Small organic molecule
Emp. Form.:
C116H163ClN30O20
Mol. Mass.:
2333.177
SMILES:
[Cl-].CCN(CC)c1ccc2c(-c3ccccc3C(=O)N3CCN(CC3)C(=O)CCC(=O)N[C@H](C\C=C/C[C@@H](N)C(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc3ccc(O)cc3)C(N)=O)C(=O)N[C@@H](Cc3ccc(O)cc3)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc3ccc(O)cc3)C(N)=O)c3ccc(cc3oc2c1)=[N+](CC)CC |r,wU:42.43,65.67,84.86,99.102,122.126,141.145,32.33,wD:54.56,73.75,111.115,130.134,37.37,(18.79,-12.44,;12.91,-.41,;12.89,-1.94,;11.55,-2.69,;10.23,-1.91,;10.25,-.37,;11.53,-4.22,;10.18,-4.98,;10.16,-6.52,;11.48,-7.3,;11.47,-8.83,;10.14,-9.58,;8.81,-8.8,;7.48,-9.56,;7.48,-11.1,;8.8,-11.87,;10.12,-11.1,;11.44,-11.88,;12.1,-10.37,;11.43,-13.41,;12.76,-14.19,;12.75,-15.71,;11.42,-16.46,;10.09,-15.71,;10.1,-14.17,;11.41,-18,;10.08,-18.76,;12.74,-18.77,;12.74,-20.3,;14.06,-21.07,;15.39,-20.3,;14.06,-22.6,;15.39,-23.37,;15.39,-24.91,;14.06,-25.68,;14.06,-27.22,;15.39,-27.99,;15.39,-29.53,;14.06,-30.3,;16.73,-30.3,;16.73,-31.84,;18.06,-29.53,;19.43,-30.23,;20.72,-29.39,;22.1,-30.08,;22.18,-31.62,;23.56,-32.32,;24.85,-31.47,;26.23,-32.16,;24.76,-29.93,;23.38,-29.24,;19.52,-31.77,;18.23,-32.61,;20.89,-32.47,;20.98,-34.01,;19.7,-34.85,;19.78,-36.39,;18.49,-37.23,;18.57,-38.77,;19.94,-39.46,;20.03,-40.99,;21.22,-38.63,;22.35,-34.7,;23.64,-33.86,;22.45,-36.24,;23.81,-36.93,;25.1,-36.09,;26.48,-36.78,;27.77,-35.94,;26.57,-38.32,;23.91,-38.47,;22.62,-39.31,;25.28,-39.16,;25.37,-40.7,;24.08,-41.55,;24.16,-43.08,;22.87,-43.93,;22.96,-45.47,;21.67,-46.31,;20.29,-45.61,;21.76,-47.85,;26.74,-41.4,;28.03,-40.55,;26.83,-42.94,;28.2,-43.64,;29.49,-42.79,;30.87,-43.48,;30.95,-45.02,;32.33,-45.71,;33.62,-44.87,;35,-45.57,;33.54,-43.33,;32.16,-42.64,;28.29,-45.17,;29.67,-45.87,;27,-46.01,;16.73,-22.6,;16.73,-21.06,;18.06,-23.37,;18.74,-21.99,;17.88,-20.71,;18.55,-19.32,;20.09,-19.22,;20.77,-17.83,;19.91,-16.55,;20.57,-15.16,;18.36,-16.66,;17.69,-18.05,;20.27,-21.88,;21.14,-23.15,;20.95,-20.49,;22.49,-20.38,;23.35,-21.66,;24.89,-21.55,;25.75,-22.83,;27.29,-22.72,;28.16,-24,;27.48,-25.38,;29.69,-23.89,;23.16,-19,;22.3,-17.72,;24.7,-18.89,;25.38,-17.51,;24.51,-16.23,;25.19,-14.84,;24.33,-13.56,;26.73,-14.74,;26.91,-17.39,;27.78,-18.67,;27.59,-16.01,;29.13,-15.9,;29.99,-17.18,;31.52,-17.07,;32.38,-18.35,;33.93,-18.24,;34.79,-19.52,;34.11,-20.9,;36.33,-19.4,;29.8,-14.52,;28.94,-13.24,;31.34,-14.41,;32.02,-13.02,;31.15,-11.75,;31.82,-10.36,;33.37,-10.25,;34.03,-8.87,;33.17,-7.59,;33.85,-6.2,;31.64,-7.69,;30.96,-9.08,;33.55,-12.91,;34.23,-11.53,;34.41,-14.19,;12.79,-9.6,;12.76,-11.12,;14.07,-11.9,;15.41,-11.15,;15.42,-9.64,;14.12,-8.86,;14.14,-7.33,;12.82,-6.55,;12.85,-5.01,;16.72,-11.93,;16.71,-13.47,;15.38,-14.22,;18.06,-11.18,;18.07,-9.65,)|