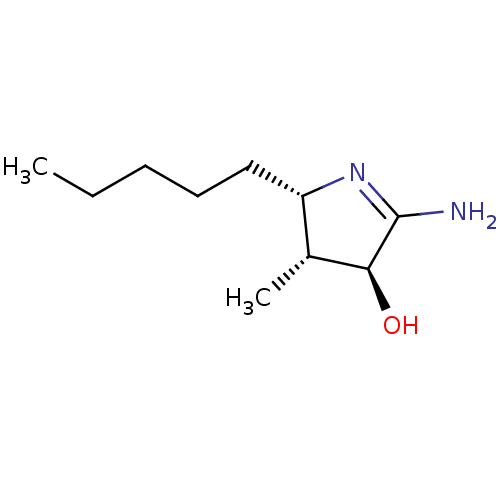
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Nitric oxide synthase, inducible
Ligand
BDBM50120681
Substrate
n/a
Meas. Tech.
ChEBML_89198
IC50
1800±n/a nM
Citation
 Tsymbalov, S; Hagen, TJ; Moore, WM; Jerome, GM; Connor, JR; Manning, PT; Pitzele, BS; Hallinan, EA 3-Hydroxy-4-methyl-5-pentyl-2-iminopyrrolidine: a potent and highly selective inducible nitric oxide synthase inhibitor. Bioorg Med Chem Lett 12:3337-9 (2002) [PubMed] Article
Tsymbalov, S; Hagen, TJ; Moore, WM; Jerome, GM; Connor, JR; Manning, PT; Pitzele, BS; Hallinan, EA 3-Hydroxy-4-methyl-5-pentyl-2-iminopyrrolidine: a potent and highly selective inducible nitric oxide synthase inhibitor. Bioorg Med Chem Lett 12:3337-9 (2002) [PubMed] Article More Info.:
Target
Name:
Nitric oxide synthase, inducible
Synonyms:
HEP-NOS | Hepatocyte NOS | Inducible NO synthase | Inducible NOS | NOS type II | NOS2 | NOS2A | NOS2_HUMAN | Nitric oxide synthase, inducible (iNOS) | iNOS
Type:
Homodimer
Mol. Mass.:
131141.95
Organism:
Homo sapiens (Human)
Description:
P35228
Residue:
1153
Sequence:
MACPWKFLFKTKFHQYAMNGEKDINNNVEKAPCATSSPVTQDDLQYHNLSKQQNESPQPLVETGKKSPESLVKLDATPLSSPRHVRIKNWGSGMTFQDTLHHKAKGILTCRSKSCLGSIMTPKSLTRGPRDKPTPPDELLPQAIEFVNQYYGSFKEAKIEEHLARVEAVTKEIETTGTYQLTGDELIFATKQAWRNAPRCIGRIQWSNLQVFDARSCSTAREMFEHICRHVRYSTNNGNIRSAITVFPQRSDGKHDFRVWNAQLIRYAGYQMPDGSIRGDPANVEFTQLCIDLGWKPKYGRFDVVPLVLQANGRDPELFEIPPDLVLEVAMEHPKYEWFRELELKWYALPAVANMLLEVGGLEFPGCPFNGWYMGTEIGVRDFCDVQRYNILEEVGRRMGLETHKLASLWKDQAVVEINIAVLHSFQKQNVTIMDHHSAAESFMKYMQNEYRSRGGCPADWIWLVPPMSGSITPVFHQEMLNYVLSPFYYYQVEAWKTHVWQDEKRRPKRREIPLKVLVKAVLFACMLMRKTMASRVRVTILFATETGKSEALAWDLGALFSCAFNPKVVCMDKYRLSCLEEERLLLVVTSTFGNGDCPGNGEKLKKSLFMLKELNNKFRYAVFGLGSSMYPRFCAFAHDIDQKLSHLGASQLTPMGEGDELSGQEDAFRSWAVQTFKAACETFDVRGKQHIQIPKLYTSNVTWDPHHYRLVQDSQPLDLSKALSSMHAKNVFTMRLKSRQNLQSPTSSRATILVELSCEDGQGLNYLPGEHLGVCPGNQPALVQGILERVVDGPTPHQTVRLEALDESGSYWVSDKRLPPCSLSQALTYFLDITTPPTQLLLQKLAQVATEEPERQRLEALCQPSEYSKWKFTNSPTFLEVLEEFPSLRVSAGFLLSQLPILKPRFYSISSSRDHTPTEIHLTVAVVTYHTRDGQGPLHHGVCSTWLNSLKPQDPVPCFVRNASGFHLPEDPSHPCILIGPGTGIAPFRSFWQQRLHDSQHKGVRGGRMTLVFGCRRPDEDHIYQEEMLEMAQKGVLHAVHTAYSRLPGKPKVYVQDILRQQLASEVLRVLHKEPGHLYVCGDVRMARDVAHTLKQLVAAKLKLNEEQVEDYFFQLKSQKRYHEDIFGAVFPYEAKKDRVAVQPSSLEMSAL