Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
5-hydroxytryptamine receptor 1A
Ligand
BDBM50057465
Substrate
n/a
Meas. Tech.
ChEMBL_953 (CHEMBL616132)
Ki
5.9±n/a nM
Citation
 Boschi, D; Tron, GC; Di Stilo, A; Fruttero, R; Gasco, A; Poggesi, E; Motta, G; Leonardi, A New potential uroselective NO-donor alpha1-antagonists. J Med Chem 46:3762-5 (2003) [PubMed] Article
Boschi, D; Tron, GC; Di Stilo, A; Fruttero, R; Gasco, A; Poggesi, E; Motta, G; Leonardi, A New potential uroselective NO-donor alpha1-antagonists. J Med Chem 46:3762-5 (2003) [PubMed] Article More Info.:
Target
Name:
5-hydroxytryptamine receptor 1A
Synonyms:
5-HT-1A | 5-HT1A | 5-hydroxytryptamine receptor 1A (5-HT-1A) | 5HT1A_HUMAN | ADRB2RL1 | ADRBRL1 | Dopamine D2 receptor and serotonin 1a receptor | G-21 | HTR1A | Serotonin receptor 1A
Type:
n/a
Mol. Mass.:
46122.49
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
422
Sequence:
MDVLSPGQGNNTTSPPAPFETGGNTTGISDVTVSYQVITSLLLGTLIFCAVLGNACVVAAIALERSLQNVANYLIGSLAVTDLMVSVLVLPMAALYQVLNKWTLGQVTCDLFIALDVLCCTSSILHLCAIALDRYWAITDPIDYVNKRTPRRAAALISLTWLIGFLISIPPMLGWRTPEDRSDPDACTISKDHGYTIYSTFGAFYIPLLLMLVLYGRIFRAARFRIRKTVKKVEKTGADTRHGASPAPQPKKSVNGESGSRNWRLGVESKAGGALCANGAVRQGDDGAALEVIEVHRVGNSKEHLPLPSEAGPTPCAPASFERKNERNAEAKRKMALARERKTVKTLGIIMGTFILCWLPFFIVALVLPFCESSCHMPTLLGAIINWLGYSNSLLNPVIYAYFNKDFQNAFKKIIKCKFCRQ
Inhibitor
Name:
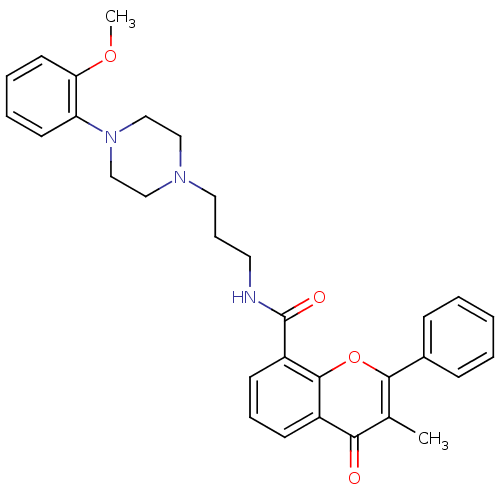
BDBM50057465
Synonyms:
3-Methyl-4-oxo-2-phenyl-4H-chromene-8-carboxylic acid {3-[4-(2-methoxy-phenyl)-piperazin-1-yl]-propyl}-amide | CHEMBL278865 | Upidosin
Type:
Small organic molecule
Emp. Form.:
C31H33N3O4
Mol. Mass.:
511.6114
SMILES:
COc1ccccc1N1CCN(CCCNC(=O)c2cccc3c2oc(c(C)c3=O)-c2ccccc2)CC1