Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Urokinase-type plasminogen activator
Ligand
BDBM50138673
Substrate
n/a
Meas. Tech.
ChEMBL_213154 (CHEMBL815981)
Ki
6.3±n/a nM
Citation
 Wendt, MD; Rockway, TW; Geyer, A; McClellan, W; Weitzberg, M; Zhao, X; Mantei, R; Nienaber, VL; Stewart, K; Klinghofer, V; Giranda, VL Identification of novel binding interactions in the development of potent, selective 2-naphthamidine inhibitors of urokinase. Synthesis, structural analysis, and SAR of N-phenyl amide 6-substitution. J Med Chem 47:303-24 (2004) [PubMed] Article
Wendt, MD; Rockway, TW; Geyer, A; McClellan, W; Weitzberg, M; Zhao, X; Mantei, R; Nienaber, VL; Stewart, K; Klinghofer, V; Giranda, VL Identification of novel binding interactions in the development of potent, selective 2-naphthamidine inhibitors of urokinase. Synthesis, structural analysis, and SAR of N-phenyl amide 6-substitution. J Med Chem 47:303-24 (2004) [PubMed] Article More Info.:
Target
Name:
Urokinase-type plasminogen activator
Synonyms:
3.4.21.73 | PLAU | U-plasminogen activator | UROK_HUMAN | Urokinase | Urokinase-type plasminogen activator (uPA) | Urokinase-type plasminogen activator chain B | Urokinase-type plasminogen activator long chain A | Urokinase-type plasminogen activator short chain A | Urokinase-type plasminogen activator/surface receptor | uPA
Type:
Enzyme
Mol. Mass.:
48528.62
Organism:
Homo sapiens (Human)
Description:
P00749
Residue:
431
Sequence:
MRALLARLLLCVLVVSDSKGSNELHQVPSNCDCLNGGTCVSNKYFSNIHWCNCPKKFGGQHCEIDKSKTCYEGNGHFYRGKASTDTMGRPCLPWNSATVLQQTYHAHRSDALQLGLGKHNYCRNPDNRRRPWCYVQVGLKLLVQECMVHDCADGKKPSSPPEELKFQCGQKTLRPRFKIIGGEFTTIENQPWFAAIYRRHRGGSVTYVCGGSLISPCWVISATHCFIDYPKKEDYIVYLGRSRLNSNTQGEMKFEVENLILHKDYSADTLAHHNDIALLKIRSKEGRCAQPSRTIQTICLPSMYNDPQFGTSCEITGFGKENSTDYLYPEQLKMTVVKLISHRECQQPHYYGSEVTTKMLCAADPQWKTDSCQGDSGGPLVCSLQGRMTLTGIVSWGRGCALKDKPGVYTRVSHFLPWIRSHTKEENGLAL
Inhibitor
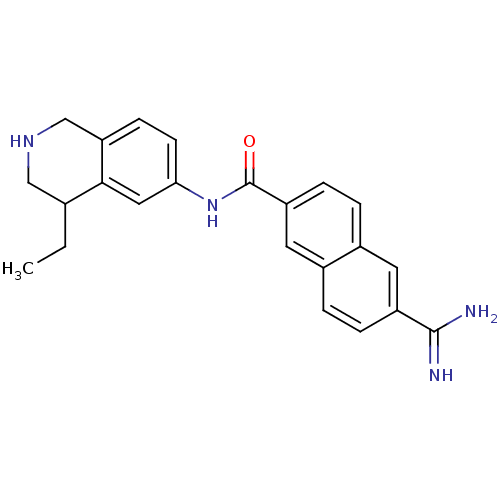
Name:
BDBM50138673
Synonyms:
6-Carbamimidoyl-naphthalene-2-carboxylic acid (4-ethyl-1,2,3,4-tetrahydro-isoquinolin-6-yl)-amide | 6-carbamimidoyl-N-(4-ethyl-1,2,3,4-tetrahydroisoquinolin-6-yl)-2-naphthamide | CHEMBL108447
Type:
Small organic molecule
Emp. Form.:
C23H24N4O
Mol. Mass.:
372.4629
SMILES:
CCC1CNCc2ccc(NC(=O)c3ccc4cc(ccc4c3)C(N)=N)cc12