Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Inhibitor of nuclear factor kappa-B kinase subunit beta
Ligand
BDBM50175698
Substrate
n/a
Meas. Tech.
ChEMBL_327452 (CHEMBL859878)
IC50
90.0±n/a nM
Citation
 Waelchli, R; Bollbuck, B; Bruns, C; Buhl, T; Eder, J; Feifel, R; Hersperger, R; Janser, P; Revesz, L; Zerwes, HG; Schlapbach, A Design and preparation of 2-benzamido-pyrimidines as inhibitors of IKK. Bioorg Med Chem Lett 16:108-12 (2005) [PubMed] Article
Waelchli, R; Bollbuck, B; Bruns, C; Buhl, T; Eder, J; Feifel, R; Hersperger, R; Janser, P; Revesz, L; Zerwes, HG; Schlapbach, A Design and preparation of 2-benzamido-pyrimidines as inhibitors of IKK. Bioorg Med Chem Lett 16:108-12 (2005) [PubMed] Article More Info.:
Target
Name:
Inhibitor of nuclear factor kappa-B kinase subunit beta
Synonyms:
I-kappa-B Kinase 2 (IKK-beta) | I-kappa-B kinase 2 | I-kappa-B-kinase beta | I-kappa-B-kinase beta (IKKB) | IKBKB | IKK-B | IKK-beta | IKK2 | IKK2/IKK1 | IKKB | IKKB_HUMAN | Inhibitor of NF-kappa-B kinase alpha/beta | Inhibitor of nuclear factor kappa B kinase beta subunit | NFKBIKB | Nuclear factor NF-kappa-B inhibitor kinase beta
Type:
Serine/threonine-protein kinase
Mol. Mass.:
86554.39
Organism:
Homo sapiens (Human)
Description:
GST-tagged IKK-2 was expressed in High Five cells and purified.
Residue:
756
Sequence:
MSWSPSLTTQTCGAWEMKERLGTGGFGNVIRWHNQETGEQIAIKQCRQELSPRNRERWCLEIQIMRRLTHPNVVAARDVPEGMQNLAPNDLPLLAMEYCQGGDLRKYLNQFENCCGLREGAILTLLSDIASALRYLHENRIIHRDLKPENIVLQQGEQRLIHKIIDLGYAKELDQGSLCTSFVGTLQYLAPELLEQQKYTVTVDYWSFGTLAFECITGFRPFLPNWQPVQWHSKVRQKSEVDIVVSEDLNGTVKFSSSLPYPNNLNSVLAERLEKWLQLMLMWHPRQRGTDPTYGPNGCFKALDDILNLKLVHILNMVTGTIHTYPVTEDESLQSLKARIQQDTGIPEEDQELLQEAGLALIPDKPATQCISDGKLNEGHTLDMDLVFLFDNSKITYETQISPRPQPESVSCILQEPKRNLAFFQLRKVWGQVWHSIQTLKEDCNRLQQGQRAAMMNLLRNNSCLSKMKNSMASMSQQLKAKLDFFKTSIQIDLEKYSEQTEFGITSDKLLLAWREMEQAVELCGRENEVKLLVERMMALQTDIVDLQRSPMGRKQGGTLDDLEEQARELYRRLREKPRDQRTEGDSQEMVRLLLQAIQSFEKKVRVIYTQLSKTVVCKQKALELLPKVEEVVSLMNEDEKTVVRLQEKRQKELWNLLKIACSKVRGPVSGSPDSMNASRLSQPGQLMSQPSTASNSLPEPAKKSEELVAEAHNLCTLLENAIQDTVREQDQSFTALDWSWLQTEEEEHSCLEQAS
Inhibitor
Name:
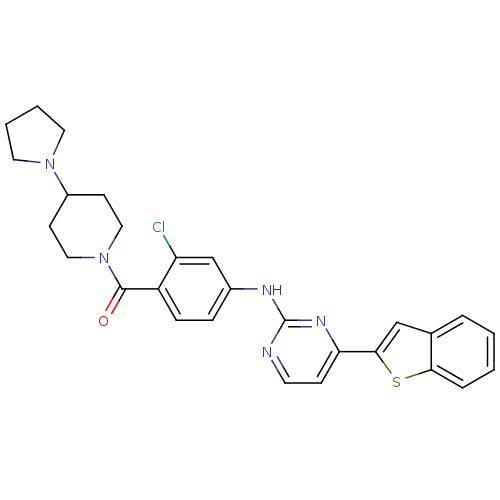
BDBM50175698
Synonyms:
(4-(4-(benzo[b]thiophen-2-yl)pyrimidin-2-ylamino)-2-chlorophenyl)(4-(pyrrolidin-1-yl)piperidin-1-yl)methanone | CHEMBL199980
Type:
Small organic molecule
Emp. Form.:
C28H28ClN5OS
Mol. Mass.:
518.073
SMILES:
Clc1cc(Nc2nccc(n2)-c2cc3ccccc3s2)ccc1C(=O)N1CCC(CC1)N1CCCC1