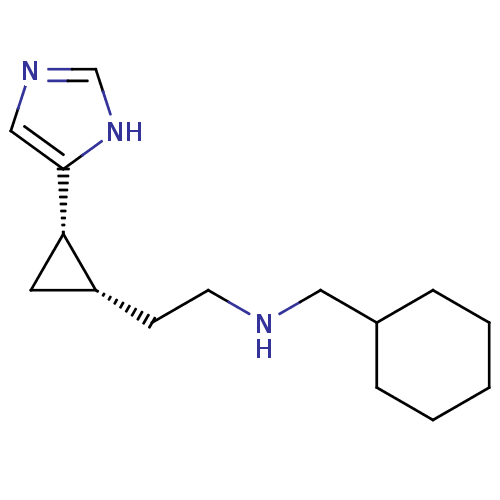
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Histamine H4 receptor
Ligand
BDBM50194211
Substrate
n/a
Meas. Tech.
ChEMBL_393875 (CHEMBL912691)
Ki
177±n/a nM
Citation
 Watanabe, M; Kazuta, Y; Hayashi, H; Yamada, S; Matsuda, A; Shuto, S Stereochemical diversity-oriented conformational restriction strategy. Development of potent histamine H3 and/or H4 receptor antagonists with an imidazolylcyclopropane structure. J Med Chem 49:5587-96 (2006) [PubMed] Article
Watanabe, M; Kazuta, Y; Hayashi, H; Yamada, S; Matsuda, A; Shuto, S Stereochemical diversity-oriented conformational restriction strategy. Development of potent histamine H3 and/or H4 receptor antagonists with an imidazolylcyclopropane structure. J Med Chem 49:5587-96 (2006) [PubMed] Article More Info.:
Target
Name:
Histamine H4 receptor
Synonyms:
AXOR35 | G-protein coupled receptor 105 | GPCR105 | GPRv53 | HH4R | HISTAMINE H4 | HRH4 | HRH4_HUMAN | Histamine H4 receptor | Histamine H4 receptor (H4R) | Histamine receptor (H3 and H4) | Pfi-013 | SP9144
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
44517.02
Organism:
Homo sapiens (Human)
Description:
Binding assays were using CHO cells stably expressing hH4R receptors.
Residue:
390
Sequence:
MPDTNSTINLSLSTRVTLAFFMSLVAFAIMLGNALVILAFVVDKNLRHRSSYFFLNLAISDFFVGVISIPLYIPHTLFEWDFGKEICVFWLTTDYLLCTASVYNIVLISYDRYLSVSNAVSYRTQHTGVLKIVTLMVAVWVLAFLVNGPMILVSESWKDEGSECEPGFFSEWYILAITSFLEFVIPVILVAYFNMNIYWSLWKRDHLSRCQSHPGLTAVSSNICGHSFRGRLSSRRSLSASTEVPASFHSERQRRKSSLMFSSRTKMNSNTIASKMGSFSQSDSVALHQREHVELLRARRLAKSLAILLGVFAVCWAPYSLFTIVLSFYSSATGPKSVWYRIAFWLQWFNSFVNPLLYPLCHKRFQKAFLKIFCIKKQPLPSQHSRSVSS