Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Maltase-glucoamylase
Ligand
BDBM50210454
Substrate
n/a
Meas. Tech.
ChEMBL_455391 (CHEMBL886166)
IC50
11000±n/a nM
Citation
More Info.:
Target
Name:
Maltase-glucoamylase
Synonyms:
Alpha glucosidase | Alpha-1,4-glucosidase | Glucan 1,4-alpha-glucosidase | MGA | MGAM | MGAML | MGA_HUMAN | Maltase | Maltase-glucoamylase, intestinal | Synonyms=MGA
Type:
Enzyme
Mol. Mass.:
209817.06
Organism:
Homo sapiens (Human)
Description:
O43451
Residue:
2753
Sequence:
MARKKLKKFTTLEIVLSVLLLVLFIISIVLIVLLAKESLKSTAPDPGTTGTPDPGTTGTPDPGTTGTTHARTTGPPDPGTTGTTPVSAECPVVNELERINCIPDQPPTKATCDQRGCCWNPQGAVSVPWCYYSKNHSYHVEGNLVNTNAGFTARLKNLPSSPVFGSNVDNVLLTAEYQTSNRFHFKLTDQTNNRFEVPHEHVQSFSGNAAASLTYQVEISRQPFSIKVTRRSNNRVLFDSSIGPLLFADQFLQLSTRLPSTNVYGLGEHVHQQYRHDMNWKTWPIFNRDTTPNGNGTNLYGAQTFFLCLEDASGLSFGVFLMNSNAMEVVLQPAPAITYRTIGGILDFYVFLGNTPEQVVQEYLELIGRPALPSYWALGFHLSRYEYGTLDNMREVVERNRAAQLPYDVQHADIDYMDERRDFTYDSVDFKGFPEFVNELHNNGQKLVIIVDPAISNNSSSSKPYGPYDRGSDMKIWVNSSDGVTPLIGEVWPGQTVFPDYTNPNCAVWWTKEFELFHNQVEFDGIWIDMNEVSNFVDGSVSGCSTNNLNNPPFTPRILDGYLFCKTLCMDAVQHWGKQYDIHNLYGYSMAVATAEAAKTVFPNKRSFILTRSTFAGSGKFAAHWLGDNTATWDDLRWSIPGVLEFNLFGIPMVGPDICGFALDTPEELCRRWMQLGAFYPFSRNHNGQGYKDQDPASFGADSLLLNSSRHYLNIRYTLLPYLYTLFFRAHSRGDTVARPLLHEFYEDNSTWDVHQQFLWGPGLLITPVLDEGAEKVMAYVPDAVWYDYETGSQVRWRKQKVEMELPGDKIGLHLRGGYIFPTQQPNTTTLASRKNPLGLIIALDENKEAKGELFWDNGETKDTVANKVYLLCEFSVTQNRLEVNISQSTYKDPNNLAFNEIKILGTEEPSNVTVKHNGVPSQTSPTVTYDSNLKVAIITDIDLLLGEAYTVEWSIKIRDEEKIDCYPDENGASAENCTARGCIWEASNSSGVPFCYFVNDLYSVSDVQYNSHGATADISLKSSVYANAFPSTPVNPLRLDVTYHKNEMLQFKIYDPNKNRYEVPVPLNIPSMPSSTPEGQLYDVLIKKNPFGIEIRRKSTGTIIWDSQLLGFTFSDMFIRISTRLPSKYLYGFGETEHRSYRRDLEWHTWGMFSRDQPPGYKKNSYGVHPYYMGLEEDGSAHGVLLLNSNAMDVTFQPLPALTYRTTGGVLDFYVFLGPTPELVTQQYTELIGRPVMVPYWSLGFQLCRYGYQNDSEIASLYDEMVAAQIPYDVQYSDIDYMERQLDFTLSPKFAGFPALINRMKADGMRVILILDPAISGNETQPYPAFTRGVEDDVFIKYPNDGDIVWGKVWPDFPDVVVNGSLDWDSQVELYRAYVAFPDFFRNSTAKWWKREIEELYNNPQNPERSLKFDGMWIDMNEPSSFVNGAVSPGCRDASLNHPPYMPHLESRDRGLSSKTLCMESQQILPDGSLVQHYNVHNLYGWSQTRPTYEAVQEVTGQRGVVITRSTFPSSGRWAGHWLGDNTAAWDQLKKSIIGMMEFSLFGISYTGADICGFFQDAEYEMCVRWMQLGAFYPFSRNHNTIGTRRQDPVSWDAAFVNISRNVLQTRYTLLPYLYTLMQKAHTEGVTVVRPLLHEFVSDQVTWDIDSQFLLGPAFLVSPVLERNARNVTAYFPRARWYDYYTGVDINARGEWKTLPAPLDHINLHVRGGYILPWQEPALNTHLSRKNPLGLIIALDENKEAKGELFWDDGQTKDTVAKKVYLLCEFSVTQNHLEVTISQSTYKDPNNLAFNEIKILGMEEPSNVTVKHNGVPSQTSPTVTYDSNLKVAIITDINLFLGEAYTVEWSIKIRDEEKIDCYPDENGDSAENCTARGCIWEASNSSGVPFCYFVNDLYSVSDVQYNSHGATADISLKSSVHANAFPSTPVNPLRLDVTYHKNEMLQFKIYDPNNNRYEVPVPLNIPSVPSSTPEGQLYDVLIKKNPFGIEIRRKSTGTIIWDSQLLGFTFNDMFIRISTRLPSKYLYGFGETEHTSYRRDLEWHTWGMFSRDQPPGYKKNSYGVHPYYMGLEEDGSAHGVLLLNSNAMDVTFQPLPALTYRTTGGVLDFYVFLGPTPELVTQQYTELIGRPVMVPYWSLGFQLCRYGYQNDSEISSLYDEMVAAQIPYDVQYSDIDYMERQLDFTLSPKFAGFPALINRMKADGMRVILILDPAISGNETQPYPAFTRGVEDDVFIKYPNDGDIVWGKVWPDFPDVVVNGSLDWDSQVELYRAYVAFPDFFRNSTAKWWKREIEELYNNPQNPERSLKFDGMWIDMNEPSSFVNGAVSPGCRDASLNHPPYMPYLESRDRGLSSKTLCMESQQILPDGSPVQHYNVHNLYGWSQTRPTYEAVQEVTGQRGVVITRSTFPSSGRWAGHWLGDNTAAWDQLKKSIIGMMEFSLFGISYTGADICGFFQDAEYEMCVRWMQLGAFYPFSRNHNTIGTRRQDPVSWDVAFVNISRTVLQTRYTLLPYLYTLMHKAHTEGVTVVRPLLHEFVSDQVTWDIDSQFLLGPAFLVSPVLERNARNVTAYFPRARWYDYYTGVDINARGEWKTLPAPLDHINLHVRGGYILPWQEPALNTHLSRQKFMGFKIALDDEGTAGGWLFWDDGQSIDTYGKGLYYLASFSASQNTMQSHIIFNNYITGTNPLKLGYIEIWGVGSVPVTSVSISVSGMVITPSFNNDPTTQVLSIDVTDRNISLHNFTSLTWISTL
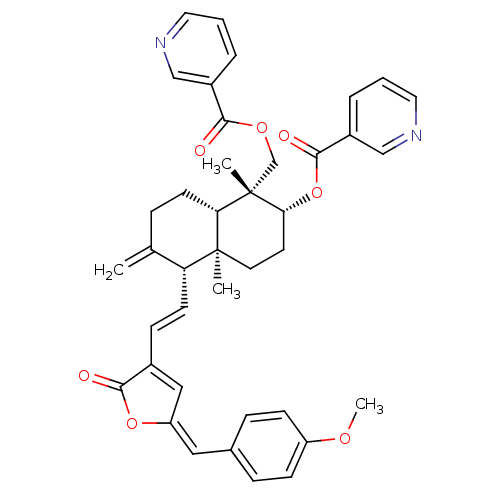
Inhibitor
Name:
BDBM50210454
Synonyms:
14-deoxy-11,12-didehydro-3,19-dinicotinate-15-p-methoxylbenzylidene andrographolide | CHEMBL394049
Type:
Small organic molecule
Emp. Form.:
C40H40N2O7
Mol. Mass.:
660.7548
SMILES:
COc1ccc(\C=C2\OC(=O)C(\C=C\[C@@H]3C(=C)CC[C@@H]4[C@](C)(COC(=O)c5cccnc5)[C@@H](CC[C@@]34C)OC(=O)c3cccnc3)=C2)cc1 |c:49|