Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cannabinoid receptor 2
Ligand
BDBM50218273
Substrate
n/a
Meas. Tech.
ChEMBL_448728 (CHEMBL897874)
IC50
170±n/a nM
Citation
 Ohta, H; Ishizaka, T; Yoshinaga, M; Morita, A; Tomishima, Y; Toda, Y; Saito, S Sulfonamide derivatives as new potent and selective CB2 cannabinoid receptor agonists. Bioorg Med Chem Lett 17:5133-5 (2007) [PubMed] Article
Ohta, H; Ishizaka, T; Yoshinaga, M; Morita, A; Tomishima, Y; Toda, Y; Saito, S Sulfonamide derivatives as new potent and selective CB2 cannabinoid receptor agonists. Bioorg Med Chem Lett 17:5133-5 (2007) [PubMed] Article More Info.:
Target
Name:
Cannabinoid receptor 2
Synonyms:
CANNABINOID CB2 | CB-2 | CB2 | CB2A | CB2B | CNR2 | CNR2_HUMAN | CX5 | Cannabinoid CB2 receptor | Cannabinoid receptor 2 (CB2) | Cannabinoid receptor 2 (CB2R) | hCB2
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
39690.94
Organism:
Homo sapiens (Human)
Description:
P34972
Residue:
360
Sequence:
MEECWVTEIANGSKDGLDSNPMKDYMILSGPQKTAVAVLCTLLGLLSALENVAVLYLILSSHQLRRKPSYLFIGSLAGADFLASVVFACSFVNFHVFHGVDSKAVFLLKIGSVTMTFTASVGSLLLTAIDRYLCLRYPPSYKALLTRGRALVTLGIMWVLSALVSYLPLMGWTCCPRPCSELFPLIPNDYLLSWLLFIAFLFSGIIYTYGHVLWKAHQHVASLSGHQDRQVPGMARMRLDVRLAKTLGLVLAVLLICWFPVLALMAHSLATTLSDQVKKAFAFCSMLCLINSMVNPVIYALRSGEIRSSAHHCLAHWKKCVRGLGSEAKEEAPRSSVTETEADGKITPWPDSRDLDLSDC
Inhibitor
Name:
BDBM50218273
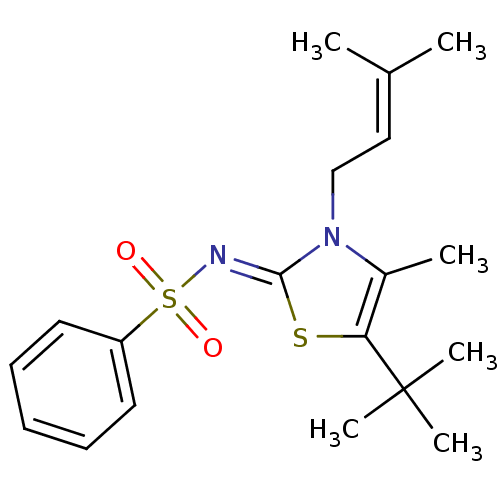
Synonyms:
(Z)-N-(5-tert-butyl-4-methyl-3-(3-methylbut-2-enyl)thiazol-2(3H)-ylidene)benzenesulfonamide | CHEMBL399219
Type:
Small organic molecule
Emp. Form.:
C19H26N2O2S2
Mol. Mass.:
378.552
SMILES:
CC(C)=CCn1c(C)c(s\c1=N/S(=O)(=O)c1ccccc1)C(C)(C)C |(19.4,-19.66,;18.07,-20.44,;16.73,-19.68,;18.08,-21.98,;16.75,-22.76,;16.77,-24.3,;15.3,-24.77,;14.06,-23.86,;15.29,-26.31,;16.75,-26.8,;17.66,-25.55,;19.2,-25.55,;19.97,-26.89,;18.64,-27.64,;21.31,-26.12,;20.74,-28.22,;19.97,-29.55,;20.74,-30.88,;22.28,-30.88,;23.05,-29.53,;22.27,-28.21,;13.93,-27.06,;12.58,-27.8,;13.19,-25.71,;14.67,-28.41,)|