Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Acyl-CoA:cholesterol acyltransferase
Ligand
BDBM50284972
Substrate
n/a
Meas. Tech.
ChEBML_28195
IC50
20±n/a nM
Citation
 Kumazawa, T; Harakawa, H; Fukui, H; Shirakura, S; Ohishi, E; Yamada, K N-(1-phenyl-2-benzimidazolyl)-N′-phenylurea derivatives as potent in hibitors of acylcoa:cholesterol acyltransferase (ACAT) Bioorg Med Chem Lett 5:1829-1832 (1995) Article
Kumazawa, T; Harakawa, H; Fukui, H; Shirakura, S; Ohishi, E; Yamada, K N-(1-phenyl-2-benzimidazolyl)-N′-phenylurea derivatives as potent in hibitors of acylcoa:cholesterol acyltransferase (ACAT) Bioorg Med Chem Lett 5:1829-1832 (1995) Article More Info.:
Target
Name:
Acyl-CoA:cholesterol acyltransferase
Synonyms:
ACAT
Type:
n/a
Mol. Mass.:
35405.31
Organism:
Oryctolagus cuniculus
Description:
n/a
Residue:
305
Sequence:
PLFLKEVGSHFDDFVTNLIEKSASLDNGGCALTTFSILKEMKNNHRAKDLRAPPEQGKIFVARRSLLDELFEVDHIRTIYHMFIALLILFILSTLVVDYIDEGRLVLEFNLLSYAFGKLPTVVWTWWTMFLSTLSIPYFLFQHWANGYSKSSHPLMYSLFHGLLFMVFQLGILGFGPTYIVLAYTLPPASRFIVILEQIRLIMKAHSFVRENVPRVLNSAKEKSSTVPIPTVNQYLYFLFAPTLIYRDSYPRTPTVRWGYVAMQFAQVFGCLFYVYYIFERLCAPLFRNIKQEPFSARVLVLCIF
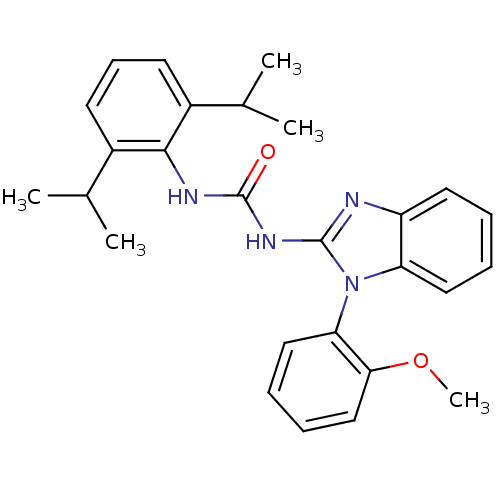
Inhibitor
Name:
BDBM50284972
Synonyms:
1-(2,6-Diisopropyl-phenyl)-3-[1-(2-methoxy-phenyl)-1H-benzoimidazol-2-yl]-urea | CHEMBL53537
Type:
Small organic molecule
Emp. Form.:
C27H30N4O2
Mol. Mass.:
442.5527
SMILES:
COc1ccccc1-n1c(NC(=O)Nc2c(cccc2C(C)C)C(C)C)nc2ccccc12 |(7.05,-13.56,;7.82,-12.23,;9.36,-12.23,;9.36,-13.75,;10.69,-14.54,;12.02,-13.77,;12.02,-12.23,;10.72,-11.46,;10.72,-9.92,;11.63,-8.68,;13.17,-8.66,;13.93,-7.33,;13.14,-6,;15.47,-7.33,;16.22,-5.98,;17.76,-5.98,;18.53,-4.64,;17.76,-3.31,;16.2,-3.32,;15.45,-4.67,;13.91,-4.67,;13.12,-3.34,;12.42,-5.07,;18.53,-7.31,;20.07,-7.3,;17.78,-8.64,;10.71,-7.43,;9.25,-7.92,;7.91,-7.15,;6.58,-7.93,;6.58,-9.47,;7.91,-10.24,;9.25,-9.46,)|