Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Prostaglandin G/H synthase 2
Ligand
BDBM17638
Substrate
n/a
Meas. Tech.
ChEBML_157856
IC50
10±n/a nM
Citation
 Black, WC; Bayly, C; Belley, M; Chan, CC; Charleson, S; Denis, D; Gauthier, JY; Gordon, R; Guay, D; Kargman, S; Lau, CK; Leblanc, Y; Mancini, J; Ouellet, M; Percival, D; Roy, P; Skorey, K; Tagari, P; Vickers, P; Wong, E From indomethacin to a selective COX-2 inhibitor: Development of indolalkanoic acids as potent and selective cyclooxygenase-2 inhibitors Bioorg Med Chem Lett 6:725-730 (1996) Article
Black, WC; Bayly, C; Belley, M; Chan, CC; Charleson, S; Denis, D; Gauthier, JY; Gordon, R; Guay, D; Kargman, S; Lau, CK; Leblanc, Y; Mancini, J; Ouellet, M; Percival, D; Roy, P; Skorey, K; Tagari, P; Vickers, P; Wong, E From indomethacin to a selective COX-2 inhibitor: Development of indolalkanoic acids as potent and selective cyclooxygenase-2 inhibitors Bioorg Med Chem Lett 6:725-730 (1996) Article More Info.:
Target
Name:
Prostaglandin G/H synthase 2
Synonyms:
Cox-2 | Cox2 | Cyclooxygenase | PGH2_RAT | Ptgs2
Type:
Enzyme Catalytic Domain
Mol. Mass.:
69173.51
Organism:
RAT
Description:
COX-2 0 RAT::P35355
Residue:
604
Sequence:
MLFRAVLLCAALALSHAANPCCSNPCQNRGECMSIGFDQYKCDCTRTGFYGENCTTPEFLTRIKLLLKPTPNTVHYILTHFKGVWNIVNNIPFLRNSIMRYVLTSRSHLIDSPPTYNVHYGYKSWEAFSNLSYYTRALPPVADDCPTPMGVKGNKELPDSKEVLEKVLLRREFIPDPQGTNMMFAFFAQHFTHQFFKTDQKRGPGFTRGLGHGVDLNHVYGETLDRQHKLRLFQDGKLKYQVIGGEVYPPTVKDTQVDMIYPPHVPEHLRFAVGQEVFGLVPGLMMYATIWLREHNRVCDILKQEHPEWDDERLFQTSRLILIGETIKIVIEDYVQHLSGYHFKLKFDPELLFNQQFQYQNRIASEFNTLYHWHPLLPDTFNIEDQEYTFKQFLYNNSILLEHGLAHFVESFTRQIAGRVAGGRNVPIAVQAVAKASIDQSREMKYQSLNEYRKRFSLKPYTSFEELTGEKEMAAELKALYHDIDAMELYPALLVEKPRPDAIFGETMVELGAPFSLKGLMGNPICSPQYWKPSTFGGEVGFRIINTASIQSLICNNVKGCPFASFNVQDPQPTKTATINASASHSRLDDINPTVLIKRRSTEL
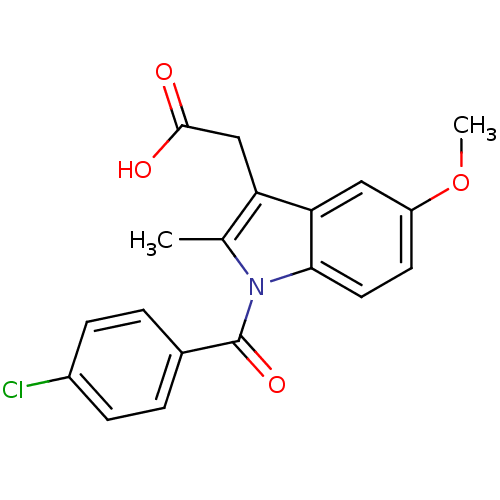
Inhibitor
Name:
BDBM17638
Synonyms:
2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl-1H-indol-3-yl}acetic acid | CHEMBL6 | Indocin | Indomethacin | US11478464, Compound Indomethacin | US11786535, Compound Indomethacin | US9271961, Indomethacin | indometacin
Type:
Small organic molecule
Emp. Form.:
C19H16ClNO4
Mol. Mass.:
357.788
SMILES:
COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1