Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50314108
Substrate
n/a
Meas. Tech.
ChEMBL_627653 (CHEMBL1114483)
IC50
>100000±n/a nM
Citation
 Debenham, JS; Madsen-Duggan, CB; Toupence, RB; Walsh, TF; Wang, J; Tong, X; Kumar, S; Lao, J; Fong, TM; Xiao, JC; Huang, CR; Shen, CP; Feng, Y; Marsh, DJ; Stribling, DS; Shearman, LP; Strack, AM; Goulet, MT Furo[2,3-b]pyridine-based cannabinoid-1 receptor inverse agonists: synthesis and biological evaluation. Part 1. Bioorg Med Chem Lett 20:1448-52 (2010) [PubMed] Article
Debenham, JS; Madsen-Duggan, CB; Toupence, RB; Walsh, TF; Wang, J; Tong, X; Kumar, S; Lao, J; Fong, TM; Xiao, JC; Huang, CR; Shen, CP; Feng, Y; Marsh, DJ; Stribling, DS; Shearman, LP; Strack, AM; Goulet, MT Furo[2,3-b]pyridine-based cannabinoid-1 receptor inverse agonists: synthesis and biological evaluation. Part 1. Bioorg Med Chem Lett 20:1448-52 (2010) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
Inhibitor
Name:
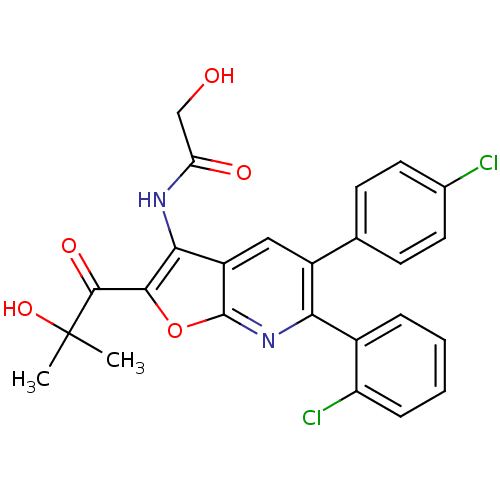
BDBM50314108
Synonyms:
CHEMBL1092169 | N-(6-(2-chlorophenyl)-5-(4-chlorophenyl)-2-(2-hydroxy-2-methylpropanoyl)furo[2,3-b]pyridin-3-yl)-2-hydroxyacetamide
Type:
Small organic molecule
Emp. Form.:
C25H20Cl2N2O5
Mol. Mass.:
499.343
SMILES:
CC(C)(O)C(=O)c1oc2nc(-c3ccccc3Cl)c(cc2c1NC(=O)CO)-c1ccc(Cl)cc1