Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
RAC-alpha serine/threonine-protein kinase
Ligand
BDBM50327912
Substrate
n/a
Meas. Tech.
ChEMBL_665069 (CHEMBL1260179)
IC50
>10000±n/a nM
Citation
 Bindi, S; Fancelli, D; Alli, C; Berta, D; Bertrand, JA; Cameron, AD; Cappella, P; Carpinelli, P; Cervi, G; Croci, V; D'Anello, M; Forte, B; Giorgini, ML; Marsiglio, A; Moll, J; Pesenti, E; Pittalà, V; Pulici, M; Riccardi-Sirtori, F; Roletto, F; Soncini, C; Storici, P; Varasi, M; Volpi, D; Zugnoni, P; Vianello, P Thieno[3,2-c]pyrazoles: a novel class of Aurora inhibitors with favorable antitumor activity. Bioorg Med Chem 18:7113-20 (2010) [PubMed] Article
Bindi, S; Fancelli, D; Alli, C; Berta, D; Bertrand, JA; Cameron, AD; Cappella, P; Carpinelli, P; Cervi, G; Croci, V; D'Anello, M; Forte, B; Giorgini, ML; Marsiglio, A; Moll, J; Pesenti, E; Pittalà, V; Pulici, M; Riccardi-Sirtori, F; Roletto, F; Soncini, C; Storici, P; Varasi, M; Volpi, D; Zugnoni, P; Vianello, P Thieno[3,2-c]pyrazoles: a novel class of Aurora inhibitors with favorable antitumor activity. Bioorg Med Chem 18:7113-20 (2010) [PubMed] Article More Info.:
Target
Name:
RAC-alpha serine/threonine-protein kinase
Synonyms:
AKT phosphorylation (p-AKT) | AKT1 | AKT1/PPP1CA | AKT1_HUMAN | C-AKT | PKB | PKB alpha | Protein kinase Akt-1 | Protein kinase B | Protein kinase B (AKT1) | Protein kinase B (Akt 1) | Protein kinase B (Akt) | Protein kinase B alpha | Protein kinase B alpha (AKT1) | Proto-oncogene Akt (Akt1) | Proto-oncogene c-Akt (AKT) | Proto-oncogene c-Akt (AKT1) | RAC | RAC-PK-alpha | RAC-alpha serine/threonine-protein kinase (AKT) | RAC-alpha serine/threonine-protein kinase (AKT1) | RAC-alpha serine/threonine-protein kinase (pAKT)
Type:
Enzyme
Mol. Mass.:
55681.25
Organism:
Homo sapiens (Human)
Description:
P31749
Residue:
480
Sequence:
MSDVAIVKEGWLHKRGEYIKTWRPRYFLLKNDGTFIGYKERPQDVDQREAPLNNFSVAQCQLMKTERPRPNTFIIRCLQWTTVIERTFHVETPEEREEWTTAIQTVADGLKKQEEEEMDFRSGSPSDNSGAEEMEVSLAKPKHRVTMNEFEYLKLLGKGTFGKVILVKEKATGRYYAMKILKKEVIVAKDEVAHTLTENRVLQNSRHPFLTALKYSFQTHDRLCFVMEYANGGELFFHLSRERVFSEDRARFYGAEIVSALDYLHSEKNVVYRDLKLENLMLDKDGHIKITDFGLCKEGIKDGATMKTFCGTPEYLAPEVLEDNDYGRAVDWWGLGVVMYEMMCGRLPFYNQDHEKLFELILMEEIRFPRTLGPEAKSLLSGLLKKDPKQRLGGGSEDAKEIMQHRFFAGIVWQHVYEKKLSPPFKPQVTSETDTRYFDEEFTAQMITITPPDQDDSMECVDSERRPHFPQFSYSASGTA
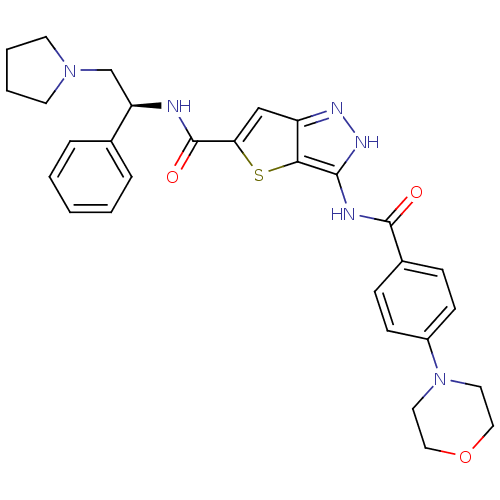
Inhibitor
Name:
BDBM50327912
Synonyms:
3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]pyrazole-5-carboxylic acid((S)-1-phenyl-2-pyrrolidin-1-yl-ethyl)-amide | CHEMBL1258913
Type:
Small organic molecule
Emp. Form.:
C29H32N6O3S
Mol. Mass.:
544.668
SMILES:
O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r|