Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Inhibitor of nuclear factor kappa-B kinase subunit epsilon
Ligand
BDBM5655
Substrate
n/a
Meas. Tech.
ChEMBL_774554 (CHEMBL1908771)
Kd
3100±n/a nM
Citation
 Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol 29:1046-51 (2011) [PubMed] Article
Davis, MI; Hunt, JP; Herrgard, S; Ciceri, P; Wodicka, LM; Pallares, G; Hocker, M; Treiber, DK; Zarrinkar, PP Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol 29:1046-51 (2011) [PubMed] Article More Info.:
Target
Name:
Inhibitor of nuclear factor kappa-B kinase subunit epsilon
Synonyms:
I kappa-B kinase epsilon | I-kappa-B Kinase 3 (IKK-epsilon) | I-kappa-B kinase epsilon (IKK-E) | I-kappa-B kinase epsilon (IKKE) | IKBKE | IKK-E | IKK-epsilon | IKK-i | IKKE | IKKE_HUMAN | IKKI | Inducible I kappa-B kinase | KIAA0151 | von Hippel-Lindau disease tumor suppressor/Inhibitor of nuclear factor kappa-B kinase subunit epsilon
Type:
Serine/threonine-protein kinase
Mol. Mass.:
80475.98
Organism:
Homo sapiens (Human)
Description:
Kinase inhibitory activity was determined using recombinant human IKK-epsilon expressed in baculovirus as a FLAG-tagged fusion protein.
Residue:
716
Sequence:
MQSTANYLWHTDDLLGQGATASVYKARNKKSGELVAVKVFNTTSYLRPREVQVREFEVLRKLNHQNIVKLFAVEETGGSRQKVLVMEYCSSGSLLSVLESPENAFGLPEDEFLVVLRCVVAGMNHLRENGIVHRDIKPGNIMRLVGEEGQSIYKLTDFGAARELDDDEKFVSVYGTEEYLHPDMYERAVLRKPQQKAFGVTVDLWSIGVTLYHAATGSLPFIPFGGPRRNKEIMYRITTEKPAGAIAGAQRRENGPLEWSYTLPITCQLSLGLQSQLVPILANILEVEQAKCWGFDQFFAETSDILQRVVVHVFSLSQAVLHHIYIHAHNTIAIFQEAVHKQTSVAPRHQEYLFEGHLCVLEPSVSAQHIAHTTASSPLTLFSTAIPKGLAFRDPALDVPKFVPKVDLQADYNTAKGVLGAGYQALRLARALLDGQELMFRGLHWVMEVLQATCRRTLEVARTSLLYLSSSLGTERFSSVAGTPEIQELKAAAELRSRLRTLAEVLSRCSQNITETQESLSSLNRELVKSRDQVHEDRSIQQIQCCLDKMNFIYKQFKKSRMRPGLGYNEEQIHKLDKVNFSHLAKRLLQVFQEECVQKYQASLVTHGKRMRVVHETRNHLRLVGCSVAACNTEAQGVQESLSKLLEELSHQLLQDRAKGAQASPPPIAPYPSPTRKDLLLHMQELCEGMKLLASDLLDNNRIIERLNRVPAPPDV
Inhibitor
Name:
BDBM5655
Synonyms:
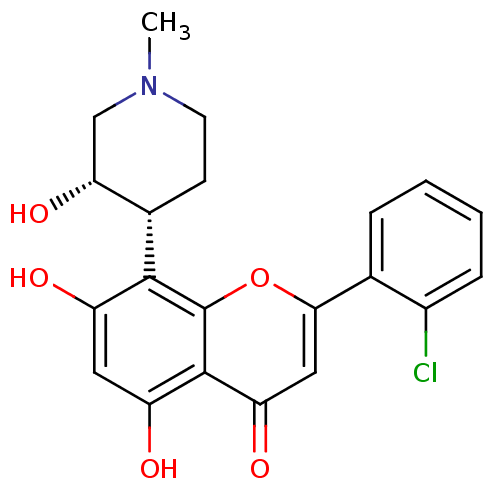
2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydroxy-1-methylpiperidin-4-yl]-4H-chromen-4-one | CHEMBL428690 | Flavopiridol | US10294218, Example Flavopiridol | US9617225, Flavopiridol
Type:
Small organic molecule
Emp. Form.:
C21H20ClNO5
Mol. Mass.:
401.84
SMILES:
CN1CC[C@@H]([C@H](O)C1)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1Cl |r|