Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
5-hydroxytryptamine receptor 4
Ligand
BDBM50161758
Substrate
n/a
Meas. Tech.
ChEMBL_3326 (CHEMBL619026)
EC50
>1000±n/a nM
Citation
 Fancelli, D; Caccia, C; Fornaretto, M; McArthur, R; Severino, D; Vaghi, F; Varasi, M Serotoninergic 5-HT3 and 5-HT4 receptor activities of dihydrobenzofuran carboxylic acid derivatives Bioorg Med Chem Lett 6:263-266 (1996) Article
Fancelli, D; Caccia, C; Fornaretto, M; McArthur, R; Severino, D; Vaghi, F; Varasi, M Serotoninergic 5-HT3 and 5-HT4 receptor activities of dihydrobenzofuran carboxylic acid derivatives Bioorg Med Chem Lett 6:263-266 (1996) Article More Info.:
Target
Name:
5-hydroxytryptamine receptor 4
Synonyms:
5-HT-4 | 5-HT4 | 5-HT4L | 5-HT4S | 5-hydroxytryptamine receptor 4 | 5HT4R_RAT | Htr4 | Serotonin (5-HT) receptor | Serotonin 4 (5-HT4) receptor
Type:
Enzyme Catalytic Domain
Mol. Mass.:
46117.31
Organism:
RAT
Description:
5-HT4 HTR4 RAT::Q62758
Residue:
406
Sequence:
MDRLDANVSSNEGFGSVEKVVLLTFFAMVILMAILGNLLVMVAVCRDRQLRKIKTNYFIVSLAFADLLVSVLVNAFGAIELVQDIWFYGEMFCLVRTSLDVLLTTASIFHLCCISLDRYYAICCQPLVYRNKMTPLRIALMLGGCWVIPMFISFLPIMQGWNNIGIVDVIEKRKFNHNSNSTFCVFMVNKPYAITCSVVAFYIPFLLMVLAYYRIYVTAKEHAQQIQMLQRAGATSESRPQTADQHSTHRMRTETKAAKTLCVIMGCFCFCWAPFFVTNIVDPFIDYTVPEKVWTAFLWLGYINSGLNPFLYAFLNKSFRRAFLIILCCDDERYKRPPILGQTVPCSTTTINGSTHVLRDTVECGGQWESRCHLTATSPLVAAQPVIRRPQDNDLEDSCSLKRSQS
Inhibitor
Name:
BDBM50161758
Synonyms:
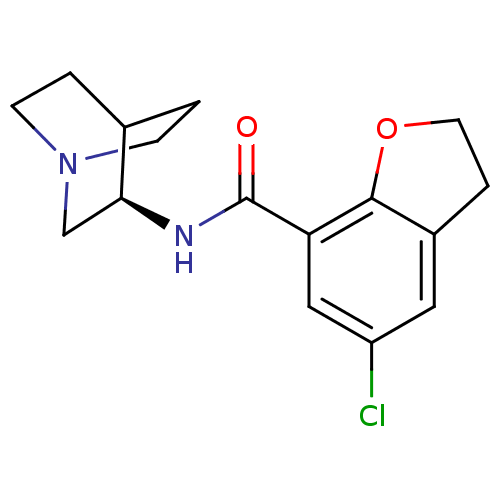
2-(5-chloro-2,3-dihydrobenzo[b]furan-7-ylcarboxamido)-(2R)-4-azabicyclo[2.2.2]octane | CHEMBL419496 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-5-chloro-2,3-dihydro-1-benzofuran-7-carboxamide
Type:
Small organic molecule
Emp. Form.:
C16H19ClN2O2
Mol. Mass.:
306.787
SMILES:
Clc1cc2CCOc2c(c1)C(=O)N[C@H]1CN2CCC1CC2 |wD:13.14,(6.37,-10.17,;7.7,-9.41,;9.02,-10.21,;10.37,-9.5,;11.82,-10.05,;12.78,-8.84,;11.91,-7.55,;10.43,-7.97,;9.14,-7.15,;7.77,-7.86,;9.21,-5.61,;7.89,-4.79,;10.56,-4.88,;10.62,-3.34,;12,-2.64,;12.07,-1.08,;10.76,-.26,;9.37,-.98,;9.31,-2.54,;10.14,-1.35,;10.78,-1.99,)|