Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Carbonic anhydrase 2
Ligand
BDBM50097280
Substrate
n/a
Meas. Tech.
ChEMBL_469183 (CHEMBL932208)
Ki
46±n/a nM
Citation
 Rami, M; Winum, JY; Innocenti, A; Montero, JL; Scozzafava, A; Supuran, CT Carbonic anhydrase inhibitors: copper(II) complexes of polyamino-polycarboxylamido aromatic/heterocyclic sulfonamides are very potent inhibitors of the tumor-associated isoforms IX and XII. Bioorg Med Chem Lett 18:836-41 (2008) [PubMed] Article
Rami, M; Winum, JY; Innocenti, A; Montero, JL; Scozzafava, A; Supuran, CT Carbonic anhydrase inhibitors: copper(II) complexes of polyamino-polycarboxylamido aromatic/heterocyclic sulfonamides are very potent inhibitors of the tumor-associated isoforms IX and XII. Bioorg Med Chem Lett 18:836-41 (2008) [PubMed] Article More Info.:
Target
Name:
Carbonic anhydrase 2
Synonyms:
CA-II | CA2 | CAC | CAH2_HUMAN | Carbonate dehydratase II | Carbonic anhydrase 2 (CA II) | Carbonic anhydrase 2 (CA-II) | Carbonic anhydrase 2 (Recombinant CA II) | Carbonic anhydrase C | Carbonic anhydrase II (CA II) | Carbonic anhydrase II (CA-II) | Carbonic anhydrase II (CAII) | Carbonic anhydrase II (hCA II) | Carbonic anhydrase isoenzyme II (hCA II)
Type:
Enzyme
Mol. Mass.:
29250.71
Organism:
Homo sapiens (Human)
Description:
P00918
Residue:
260
Sequence:
MSHHWGYGKHNGPEHWHKDFPIAKGERQSPVDIDTHTAKYDPSLKPLSVSYDQATSLRILNNGHAFNVEFDDSQDKAVLKGGPLDGTYRLIQFHFHWGSLDGQGSEHTVDKKKYAAELHLVHWNTKYGDFGKAVQQPDGLAVLGIFLKVGSAKPGLQKVVDVLDSIKTKGKSADFTNFDPRGLLPESLDYWTYPGSLTTPPLLECVTWIVLKEPISVSSEQVLKFRKLNFNGEGEPEELMVDNWRPAQPLKNRQIKASFK
Inhibitor
Name:
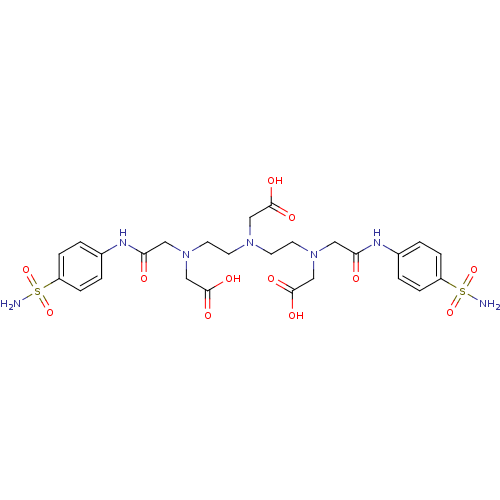
BDBM50097280
Synonyms:
CHEMBL284694 | V{{2-[Carboxymethyl-(2-{carboxymethyl-[(4-sulfamoyl-phenylcarbamoyl)-methyl]-amino}-ethyl)-amino]-ethyl}-[(4-sulfamoyl-phenylcarbamoyl)-methyl]-amino}-acetic acid | Zinc; {{2-[Carboxymethyl-(2-{carboxymethyl-[(4-sulfamoyl-phenylcarbamoyl)-methyl]-amino}-ethyl)-amino]-ethyl}-[(4-sulfamoyl-phenylcarbamoyl)-methyl]-amino}-acetic acid | {{2-[Carboxymethyl-(2-{carboxymethyl-[(4-sulfamoyl-phenylcarbamoyl)-methyl]-amino}-ethyl)-amino]-ethyl}-[(4-sulfamoyl-phenylcarbamoyl)-methyl]-amino}-acetic acid | {{2-[Carboxymethyl-(2-{carboxymethyl-[(4-sulfamoyl-phenylcarbamoyl)-methyl]-amino}-ethyl)-amino]-ethyl}-[(4-sulfamoyl-phenylcarbamoyl)-methyl]-amino}-acetic acid; compound with Zn complex
Type:
Small organic molecule
Emp. Form.:
C26H35N7O12S2
Mol. Mass.:
701.726
SMILES:
NS(=O)(=O)c1ccc(NC(=O)CN(CCN(CCN(CC(O)=O)CC(=O)Nc2ccc(cc2)S(N)(=O)=O)CC(O)=O)CC(O)=O)cc1