Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Transient receptor potential cation channel subfamily V member 1
Ligand
BDBM50333933
Substrate
n/a
Meas. Tech.
ChEMBL_700254 (CHEMBL1647472)
Ki
1.99±n/a nM
Citation
More Info.:
Target
Name:
Transient receptor potential cation channel subfamily V member 1
Synonyms:
Capsaicin receptor | OTRPC1 | TRPV1_RAT | Transient receptor potential cation channel subfamily V member 1 (TRPV1) | Trpv1 | Vanilloid Receptor 1 (TRPV1, VR1) | Vanilloid VR1 | Vanilloid receptor | Vanilloid receptor 1 (VRI/TRPV1) | Vanilloid receptor type 1-like | Vanilloid receptor type 1-like (TrpV1/Vr1) | Vr1 | Vr1l | osm-9-like TRP channel 1
Type:
Transient Receptor
Mol. Mass.:
94956.12
Organism:
Rattus norvegicus (rat)
Description:
O35433
Residue:
838
Sequence:
MEQRASLDSEESESPPQENSCLDPPDRDPNCKPPPVKPHIFTTRSRTRLFGKGDSEEASPLDCPYEEGGLASCPIITVSSVLTIQRPGDGPASVRPSSQDSVSAGEKPPRLYDRRSIFDAVAQSNCQELESLLPFLQRSKKRLTDSEFKDPETGKTCLLKAMLNLHNGQNDTIALLLDVARKTDSLKQFVNASYTDSYYKGQTALHIAIERRNMTLVTLLVENGADVQAAANGDFFKKTKGRPGFYFGELPLSLAACTNQLAIVKFLLQNSWQPADISARDSVGNTVLHALVEVADNTVDNTKFVTSMYNEILILGAKLHPTLKLEEITNRKGLTPLALAASSGKIGVLAYILQREIHEPECRHLSRKFTEWAYGPVHSSLYDLSCIDTCEKNSVLEVIAYSSSETPNRHDMLLVEPLNRLLQDKWDRFVKRIFYFNFFVYCLYMIIFTAAAYYRPVEGLPPYKLKNTVGDYFRVTGEILSVSGGVYFFFRGIQYFLQRRPSLKSLFVDSYSEILFFVQSLFMLVSVVLYFSQRKEYVASMVFSLAMGWTNMLYYTRGFQQMGIYAVMIEKMILRDLCRFMFVYLVFLFGFSTAVVTLIEDGKNNSLPMESTPHKCRGSACKPGNSYNSLYSTCLELFKFTIGMGDLEFTENYDFKAVFIILLLAYVILTYILLLNMLIALMGETVNKIAQESKNIWKLQRAITILDTEKSFLKCMRKAFRSGKLLQVGFTPDGKDDYRWCFRVDEVNWTTWNTNVGIINEDPGNCEGVKRTLSFSLRSGRVSGRNWKNFALVPLLRDASTRDRHATQQEEVQLKHYTGSLKPEDAEVFKDSMVPGEK
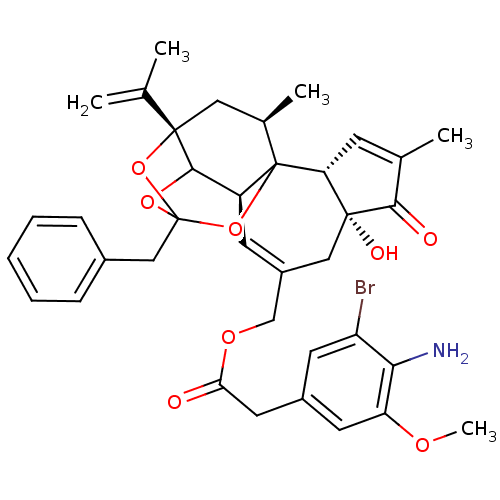
Inhibitor
Name:
BDBM50333933
Synonyms:
5-bromo-4-amino Resiniferatoxin | CHEMBL1644422
Type:
Small organic molecule
Emp. Form.:
C37H40BrNO8
Mol. Mass.:
706.619
SMILES:
COc1cc(CC(=O)OCC2=C[C@H]3C4OC5(Cc6ccccc6)O[C@@]4(C[C@@H](C)C3(O5)[C@@H]3C=C(C)C(=O)[C@@]3(O)C2)C(C)=C)cc(Br)c1N |r,t:10,35,TLB:39:24:12:29.14.15,THB:23:24:12:29.14.15,23:15:12:24.25.26,27:26:12:29.14.15|