Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Prolyl endopeptidase FAP
Ligand
BDBM50228403
Substrate
n/a
Meas. Tech.
ChEMBL_849435 (CHEMBL2149436)
IC50
89±n/a nM
Citation
 Ikuma, Y; Hochigai, H; Kimura, H; Nunami, N; Kobayashi, T; Uchiyama, K; Furuta, Y; Sakai, M; Horiguchi, M; Masui, Y; Okazaki, K; Sato, Y; Nakahira, H Discovery of 3H-imidazo[4,5-c]quinolin-4(5H)-ones as potent and selective dipeptidyl peptidase IV (DPP-4) inhibitors. Bioorg Med Chem 20:5864-83 (2012) [PubMed] Article
Ikuma, Y; Hochigai, H; Kimura, H; Nunami, N; Kobayashi, T; Uchiyama, K; Furuta, Y; Sakai, M; Horiguchi, M; Masui, Y; Okazaki, K; Sato, Y; Nakahira, H Discovery of 3H-imidazo[4,5-c]quinolin-4(5H)-ones as potent and selective dipeptidyl peptidase IV (DPP-4) inhibitors. Bioorg Med Chem 20:5864-83 (2012) [PubMed] Article More Info.:
Target
Name:
Prolyl endopeptidase FAP
Synonyms:
170 kDa melanoma membrane-bound gelatinase | FAP | Fibroblast Activation Protein (FAP) | Fibroblast activation protein alpha | Integral membrane serine protease | SEPR_HUMAN | Seprase
Type:
Enzyme
Mol. Mass.:
87712.48
Organism:
Homo sapiens (Human)
Description:
Q12884
Residue:
760
Sequence:
MKTWVKIVFGVATSAVLALLVMCIVLRPSRVHNSEENTMRALTLKDILNGTFSYKTFFPNWISGQEYLHQSADNNIVLYNIETGQSYTILSNRTMKSVNASNYGLSPDRQFVYLESDYSKLWRYSYTATYYIYDLSNGEFVRGNELPRPIQYLCWSPVGSKLAYVYQNNIYLKQRPGDPPFQITFNGRENKIFNGIPDWVYEEEMLATKYALWWSPNGKFLAYAEFNDTDIPVIAYSYYGDEQYPRTINIPYPKAGAKNPVVRIFIIDTTYPAYVGPQEVPVPAMIASSDYYFSWLTWVTDERVCLQWLKRVQNVSVLSICDFREDWQTWDCPKTQEHIEESRTGWAGGFFVSTPVFSYDAISYYKIFSDKDGYKHIHYIKDTVENAIQITSGKWEAINIFRVTQDSLFYSSNEFEEYPGRRNIYRISIGSYPPSKKCVTCHLRKERCQYYTASFSDYAKYYALVCYGPGIPISTLHDGRTDQEIKILEENKELENALKNIQLPKEEIKKLEVDEITLWYKMILPPQFDRSKKYPLLIQVYGGPCSQSVRSVFAVNWISYLASKEGMVIALVDGRGTAFQGDKLLYAVYRKLGVYEVEDQITAVRKFIEMGFIDEKRIAIWGWSYGGYVSSLALASGTGLFKCGIAVAPVSSWEYYASVYTERFMGLPTKDDNLEHYKNSTVMARAEYFRNVDYLLIHGTADDNVHFQNSAQIAKALVNAQVDFQAMWYSDQNHGLSGLSTNHLYTHMTHFLKQCFSLSD
Inhibitor
Name:
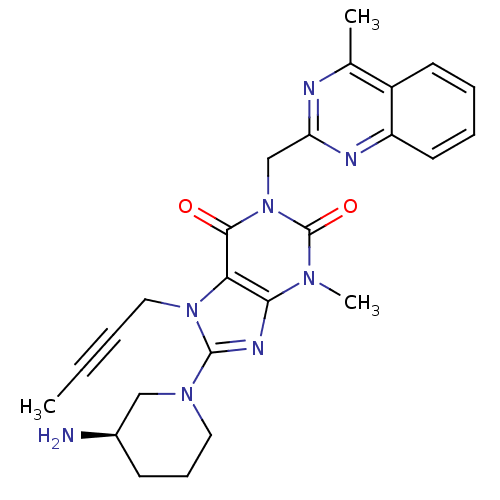
BDBM50228403
Synonyms:
(R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-methyl-1-((4-methylquinazolin-2-yl)methyl)-1H-purine-2,6(3H,7H)-dione | 8-[(3R)-3-Aminopiperidin-1-yl]-7-but-2-yn-1-yl-3-methyl-1-[(4-methylquinazolin-2-yl)methyl]-3,7-dihydro-1H-purine-2,6-dione | CHEMBL237500 | LINAGLIPTIN | US10202383, Example 2(142) | US10358449, Linagliptin | US9255098, Linagliptin | US9321791, 2(142) | US9556175, 2(142)
Type:
Small organic molecule
Emp. Form.:
C25H28N8O2
Mol. Mass.:
472.5422
SMILES:
CC#CCn1c(nc2n(C)c(=O)n(Cc3nc(C)c4ccccc4n3)c(=O)c12)N1CCC[C@@H](N)C1