Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
N-acetyllactosaminide beta-1,3-N-acetylglucosaminyltransferase 3
Ligand
BDBM50058693
Substrate
n/a
Meas. Tech.
ChEMBL_1448995 (CHEMBL3376892)
Ki
1500000±n/a nM
Citation
 Hanashima, S; Korekane, H; Taniguchi, N; Yamaguchi, Y Synthesis of N-glycan units for assessment of substrate structural requirements of N-acetylglucosaminyltransferase III. Bioorg Med Chem Lett 24:4533-7 (2014) [PubMed] Article
Hanashima, S; Korekane, H; Taniguchi, N; Yamaguchi, Y Synthesis of N-glycan units for assessment of substrate structural requirements of N-acetylglucosaminyltransferase III. Bioorg Med Chem Lett 24:4533-7 (2014) [PubMed] Article More Info.:
Target
Name:
N-acetyllactosaminide beta-1,3-N-acetylglucosaminyltransferase 3
Synonyms:
B3GALT8 | B3GALT8 | B3GN3_HUMAN | B3GNT3 | BGnT-3 | Beta-1,3-GalTase 8 | Beta-1,3-Gn-T3 | Beta-1,3-N-acetylglucosaminyltransferase 3 | Beta-1,3-galactosyl-O-glycosyl-glycoprotein beta-1,3-N-acetylglucosaminyltransferase | Beta-1,3-galactosyltransferase 8 | Beta-3-Gx-T8 | Beta3Gal-T8 | Beta3GalT8 | Beta3Gn-T3 | Core 1 extending beta-1,3-N-acetylglucosaminyltransferase | Core1-beta3GlcNAcT | N-acetyllactosaminide beta-1,3-N-acetylglucosaminyltransferase 3 | TMEM3 | Transmembrane protein 3 | UDP-Gal:beta-GlcNAc beta-1,3-galactosyltransferase 8 | UDP-GlcNAc:betaGal beta-1,3-N-acetylglucosaminyltransferase 3 | UDP-galactose:beta-N-acetylglucosamine beta-1,3-galactosyltransferase 8 | b3Gal-T8
Type:
PROTEIN
Mol. Mass.:
42548.28
Organism:
Homo sapiens (Human)
Description:
ChEMBL_109464
Residue:
372
Sequence:
MKYLRHRRPNATLILAIGAFTLLLFSLLVSPPTCKVQEQPPAIPEALAWPTPPTRPAPAPCHANTSMVTHPDFATQPQHVQNFLLYRHCRHFPLLQDVPPSKCAQPVFLLLVIKSSPSNYVRRELLRRTWGRERKVRGLQLRLLFLVGTASNPHEARKVNRLLELEAQTHGDILQWDFHDSFFNLTLKQVLFLQWQETRCANASFVLNGDDDVFAHTDNMVFYLQDHDPGRHLFVGQLIQNVGPIRAFWSKYYVPEVVTQNERYPPYCGGGGFLLSRFTAAALRRAAHVLDIFPIDDVFLGMCLELEGLKPASHSGIRTSGVRAPSQRLSSFDPCFYRDLLLVHRFLPYEMLLMWDALNQPNLTCGNQTQIY
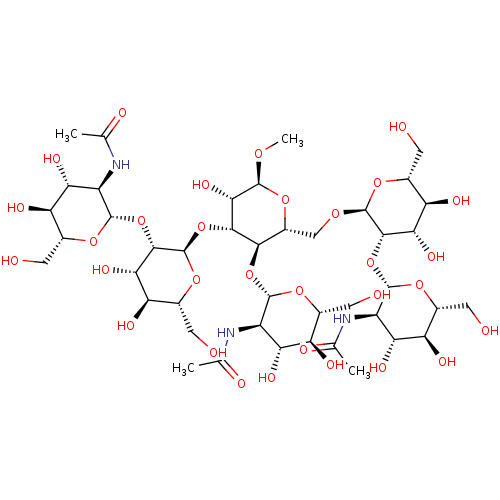
Inhibitor
Name:
BDBM50058693
Synonyms:
CHEMBL3326805
Type:
Small organic molecule
Emp. Form.:
C43H73N3O31
Mol. Mass.:
1128.0412
SMILES:
[H][C@@]1(O[C@@H]2[C@@H](CO[C@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@]3([H])O[C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3NC(C)=O)O[C@H](OC)[C@@H](O)[C@@]2([H])O[C@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@]2([H])O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2NC(C)=O)O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(C)=O |r|