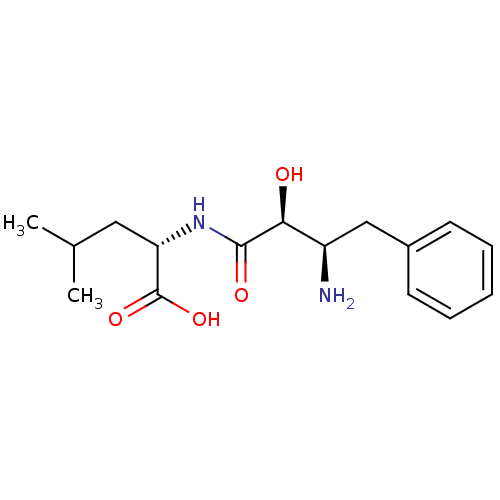
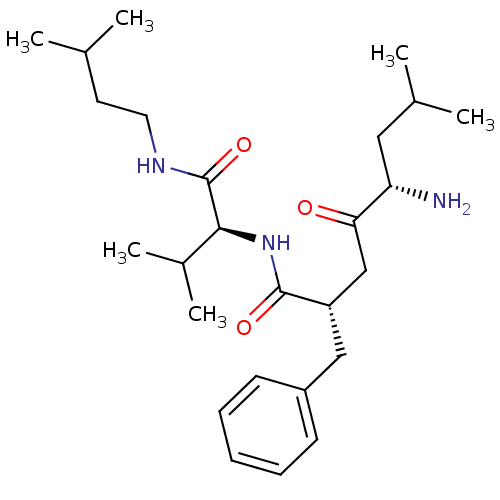
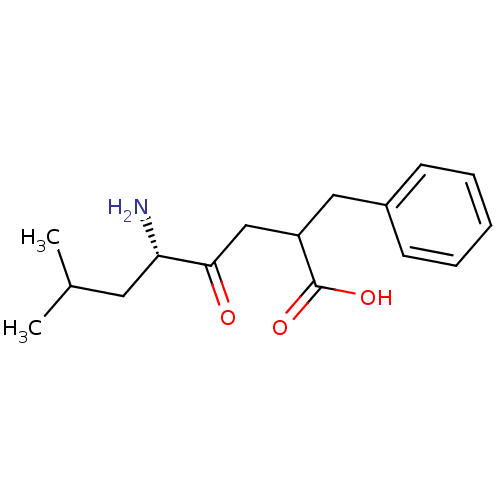
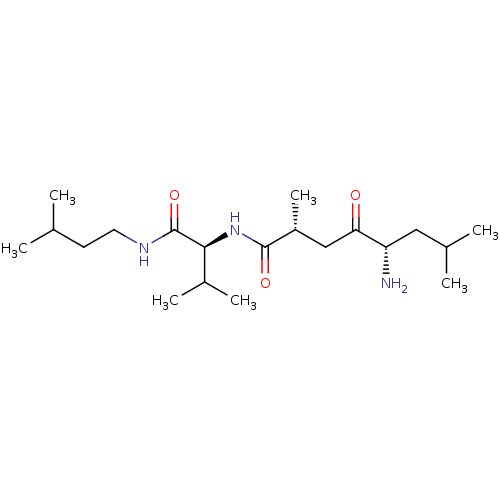
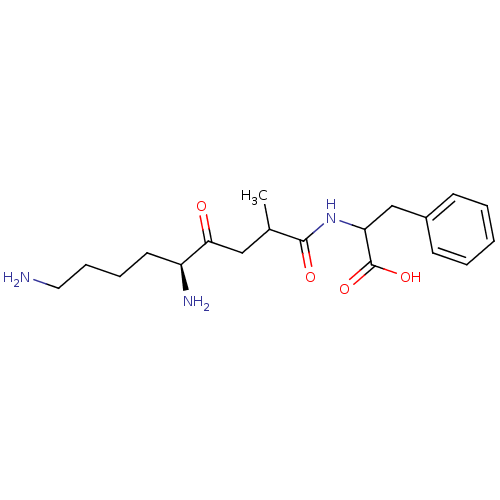
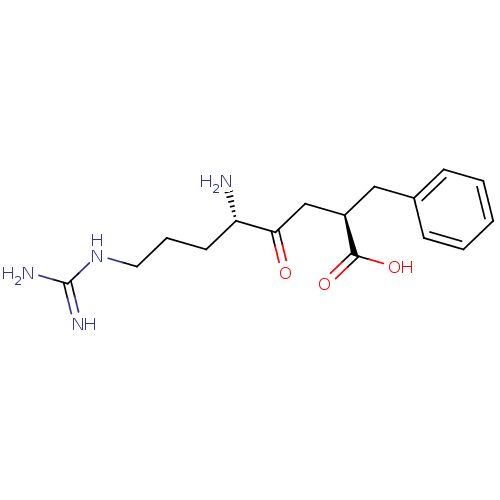
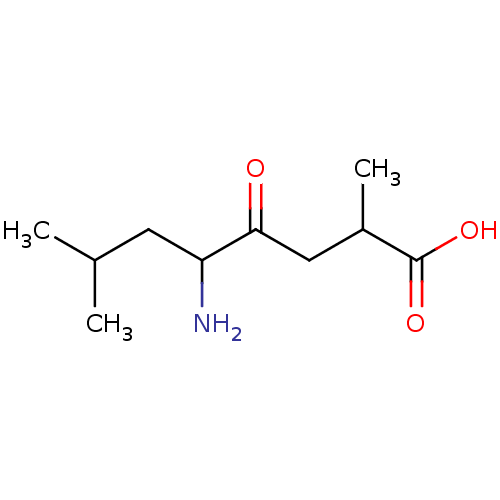
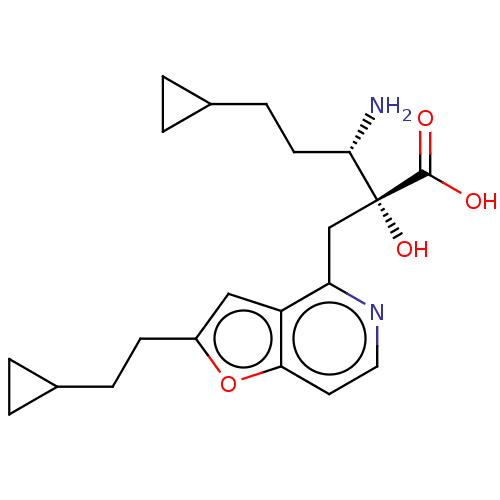
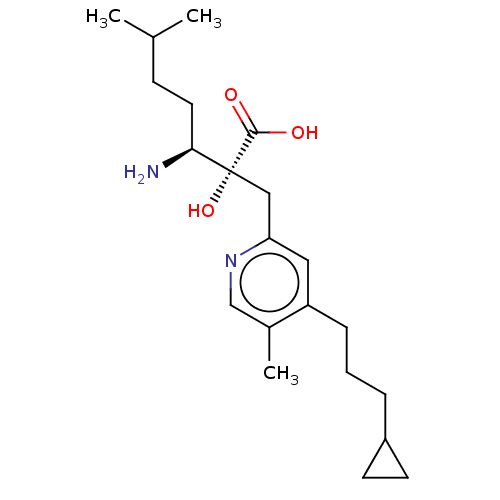
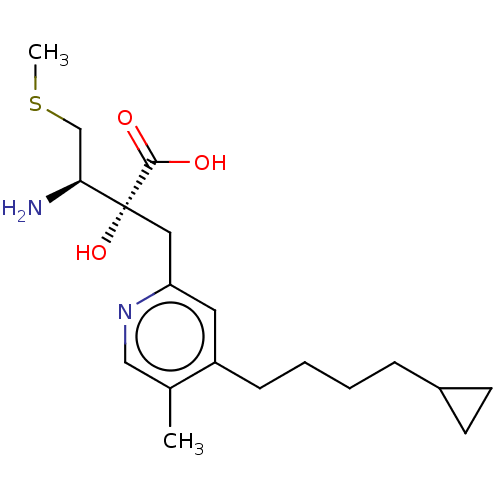
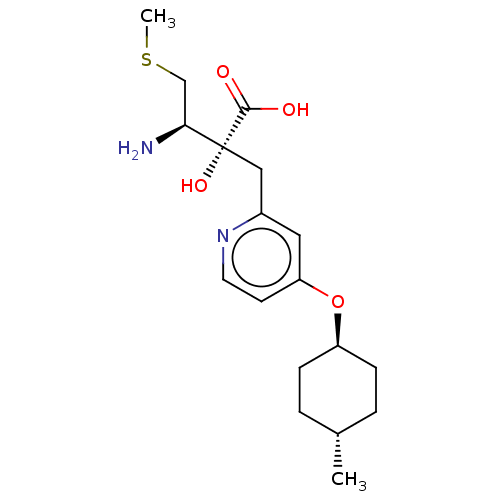
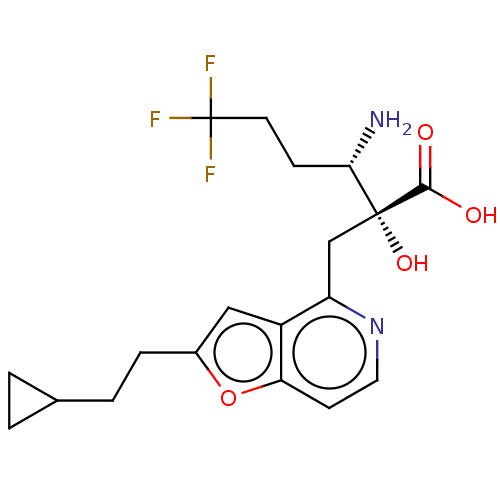
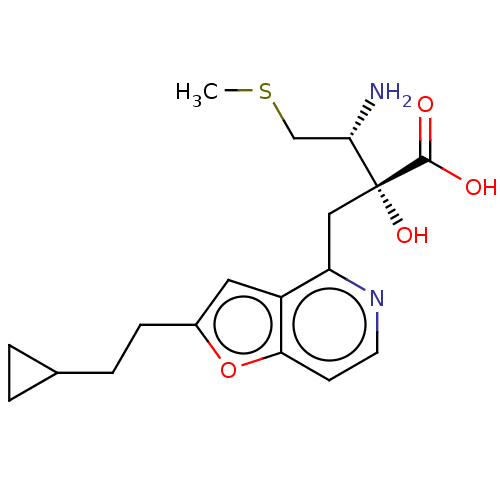
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Inhibition of leucine aminopeptidaseMore data for this Ligand-Target Pair
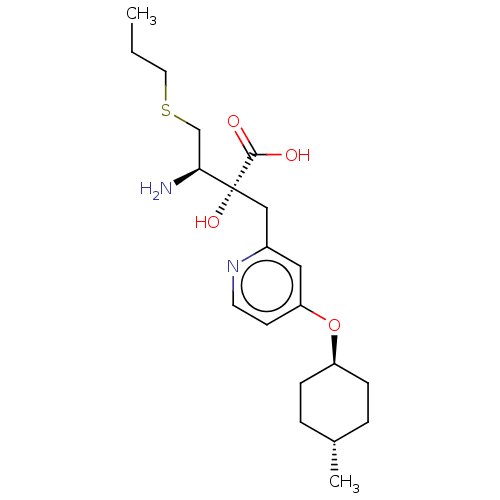
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Inhibition of aminopeptidase M or membrane leucine aminopeptidaseMore data for this Ligand-Target Pair
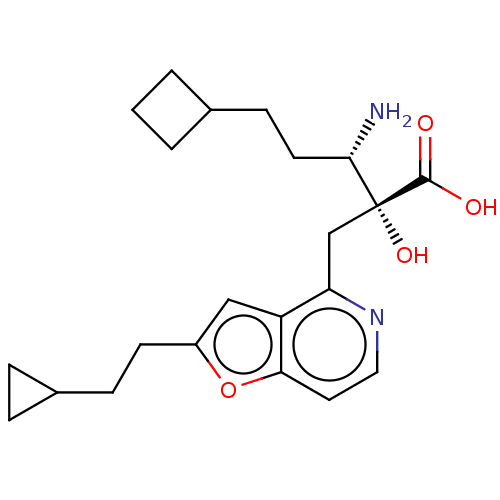
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 220nMAssay Description:Inhibition of leucine aminopeptidaseMore data for this Ligand-Target Pair
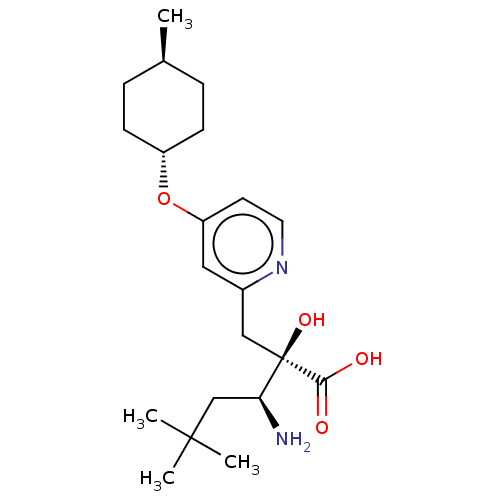
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 4.40E+3nMAssay Description:Inhibition of aminopeptidase M or membrane leucine aminopeptidaseMore data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 8.30E+3nMAssay Description:Competitive inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis)More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 2.50E+4nMAssay Description:Competitive inhibition of leucine aminopeptidase; Ki value reporting the slope effect(Kis)More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 5.70E+4nMAssay Description:Competitive inhibition of leucine aminopeptidase; Ki value reporting the slope effect(Kis)More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 7.90E+4nMAssay Description:Non-competitive inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis)More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 1.30E+5nMAssay Description:Competitive inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis)More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 1.70E+5nMAssay Description:Competitive inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis)More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 2.10E+5nMAssay Description:Inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis)More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 2.40E+5nMAssay Description:Competitive inhibition of leucine aminopeptidase; Ki value reporting the slope effect(Kis)More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 2.60E+5nMAssay Description:Inhibition of leucine aminopeptidaseMore data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: >2.97E+5nMAssay Description:Non-competitive inhibition of aminopeptidase B or arginyl aminopeptidase purified from rat liver; Ki value reporting the slope effect(Kis)More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: >3.12E+5nMAssay Description:Inhibition of leucine aminopeptidaseMore data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 3.50E+5nMAssay Description:Competitive inhibition of leucine aminopeptidase; Ki value reporting the slope effect(Kis)More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 6.60E+5nMAssay Description:Non-competitive inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the slope effect(Kis)More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 9.40E+5nMAssay Description:Non-competitive inhibition of aminopeptidase M or membrane leucine aminopeptidase; Ki value reporting the intercept effect(Kii)More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataKi: 1.10E+6nMAssay Description:Competitive inhibition of leucine aminopeptidase; Ki value reporting the slope effect(Kis)More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.420nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.420nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.560nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.580nMAssay Description:The inventors have assumed that inhibition of nocturnal activity of placental leucine aminopeptidase (P-LAP), i.e. aminopeptidase that cleaves AVP, w...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.620nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.620nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.650nMAssay Description:The inventors have assumed that inhibition of nocturnal activity of placental leucine aminopeptidase (P-LAP), i.e. aminopeptidase that cleaves AVP, w...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.770nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.770nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.770nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.770nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.910nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.910nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.920nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.920nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 0.940nMAssay Description:The inventors have assumed that inhibition of nocturnal activity of placental leucine aminopeptidase (P-LAP), i.e. aminopeptidase that cleaves AVP, w...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:IRAP: Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP....More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair
TargetLeucyl-cystinyl aminopeptidase(Rattus norvegicus)
University Of Wisconsin-Madison
Curated by ChEMBL
University Of Wisconsin-Madison
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Rat epididymal fat pads were homogenized and subjected to ultracentrifugation at 100,000×g for 30 minutes to obtain microsomes containing IRAP. The m...More data for this Ligand-Target Pair