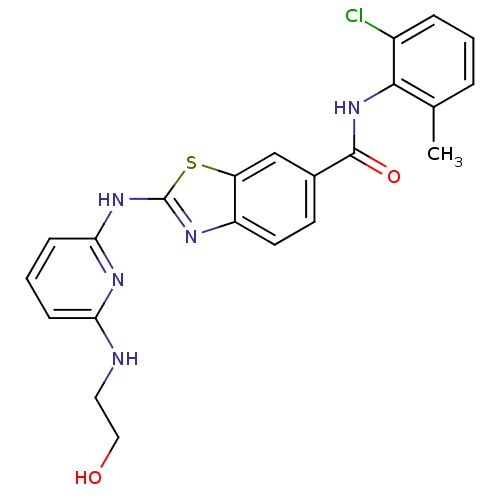
BDBM13357 CHEMBL312933::N-(2-chloro-6-methylphenyl)-2-({6-[(2-hydroxyethyl)amino]pyridin-2-yl}amino)-1,3-benzothiazole-6-carboxamide::benzothiazole analog 13a
SMILES Cc1cccc(Cl)c1NC(=O)c1ccc2nc(Nc3cccc(NCCO)n3)sc2c1
InChI Key InChIKey=RPZCFPLDPHTRPE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 13357
Found 5 hits for monomerid = 13357
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Fyn(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 2.40nMAssay Description:Inhibition of Fyn protein kinaseMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase Src(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Inhibition of Src protein tryrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of p56 Lck tyrosine kinaseMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-33P] lab...More data for this Ligand-Target Pair