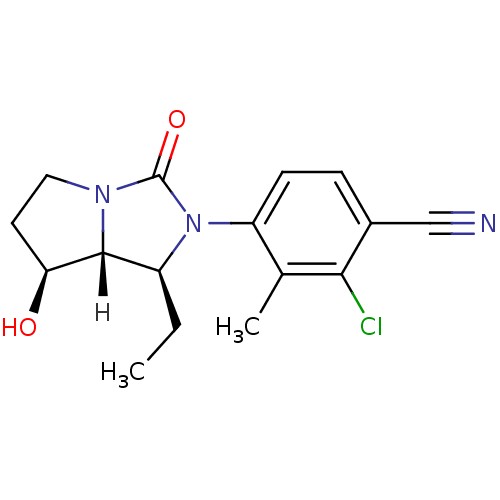
BDBM18177 4-[(1S,7S,7aR)-1-ethyl-7-hydroxy-3-oxo-hexahydro-1H-pyrrolo[1,2-a]imidazolidin-2-yl]-2-chloro-3-methylbenzonitrile::CHEMBL229861::imidazolin-2-one, 11a
SMILES [H][C@@]12[C@@H](O)CCN1C(=O)N([C@H]2CC)c1ccc(C#N)c(Cl)c1C
InChI Key InChIKey=GHMFVPOVQMEIHB-CORIIIEPSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 18177
Found 4 hits for monomerid = 18177
Affinity DataKi: 0.900nM ΔG°: -12.2kcal/mole EC50: 1.80nMpH: 7.4 T: 2°CAssay Description:Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:Agonist activity at androgen receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:Binding affinity to Androgen receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 1.80nMAssay Description:Agonist activity at Androgen receptor (unknown origin)More data for this Ligand-Target Pair