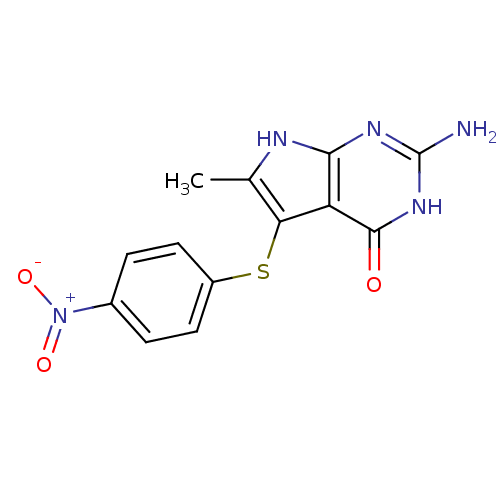
BDBM18807 2-amino-6-methyl-5-[(4-nitrophenyl)sulfanyl]-3H,4H,7H-pyrrolo[2,3-d]pyrimidin-4-one::CHEMBL400364::Pyrrolo[2,3-d]pyrimidine analogue, 2
SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)[N+]([O-])=O
InChI Key InChIKey=IXCJWBJTGZAHRW-UHFFFAOYSA-N
Data 15 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 18807
Found 15 hits for monomerid = 18807
Affinity DataIC50: >5.40E+4nMAssay Description:DHFRs were assayed spectrophotometrically in the reaction buffer solution containing dihydrofolate. The reaction was initiated with an amount of enzy...More data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibition of human thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 130nMpH: 7.4 T: 2°CAssay Description:TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi...More data for this Ligand-Target Pair
Affinity DataIC50: 4.50E+4nMpH: 7.4 T: 2°CAssay Description:TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of Escherichia coli thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.10E+3nMAssay Description:The inhibitory concentration of compound was evaluated on Lactobacillus casei Thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:The inhibitory concentration of compound was evaluated on Streptococcus faecium Thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:The inhibitory concentration of compound was evaluated on Human Thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:The inhibitory concentration of compound was evaluated on Pneumocystis carini Thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: >1.50E+4nMAssay Description:The inhibitory concentration of compound against Dihydrofolate reductases on Pneumocystis cariniMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:The inhibitory concentration of compound was evaluated on Escherichia coli Thymidylate synthaseMore data for this Ligand-Target Pair
TargetBifunctional dihydrofolate reductase-thymidylate synthase(Toxoplasma gondii)
Duquesne University
Curated by ChEMBL
Duquesne University
Curated by ChEMBL
Affinity DataIC50: 2.44E+5nMAssay Description:The inhibitory concentration of compound against Dihydrofolate reductases on Toxoplasma gondiiMore data for this Ligand-Target Pair
Affinity DataIC50: 7.47E+6nMAssay Description:The inhibitory concentration of compound against Dihydrofolate reductases on rat liverMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibitory concentration against human thymidylate synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMpH: 7.4 T: 2°CAssay Description:TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi...More data for this Ligand-Target Pair