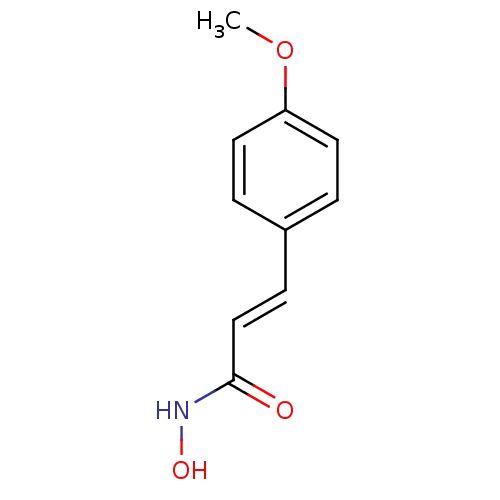
BDBM23269 (2E)-N-hydroxy-3-(4-methoxyphenyl)prop-2-enamide::Cinnamic hydroxamate deriv., 13::US10188756, Compound CN87::US11505523, Compound PCI34051
SMILES COc1ccc(\C=C\C(=O)NO)cc1
InChI Key InChIKey=BQDLTSGIXOYYHY-QPJJXVBHSA-N
Data 18 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 18 hits for monomerid = 23269
Found 18 hits for monomerid = 23269
Affinity DataIC50: 7.90E+4nMpH: 7.4 T: 2°CAssay Description:Recombinant BoNT LC/A activity was measured in black 96-well microtiter plates by use of a Molecular Devices (Sunnyvale, CA) SpectraMax GeminiEM plat...More data for this Ligand-Target Pair
Affinity DataIC50: 55.7nMAssay Description:Further provided herein are methods of inhibiting HDAC8 mediated deacetylation of p53. In one aspect, the method includes contacting HDAC8 with a HDA...More data for this Ligand-Target Pair
Affinity DataIC50: 833nMAssay Description:All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a...More data for this Ligand-Target Pair
Affinity DataIC50: 238nMAssay Description:All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a...More data for this Ligand-Target Pair
Affinity DataIC50: >7.00E+4nMAssay Description:All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a...More data for this Ligand-Target Pair
Affinity DataIC50: 23.2nMAssay Description:All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a...More data for this Ligand-Target Pair
Affinity DataIC50: >7.00E+4nMAssay Description:All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a...More data for this Ligand-Target Pair
Affinity DataIC50: 2.57E+3nMAssay Description:All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a...More data for this Ligand-Target Pair
Affinity DataIC50: >7.00E+4nMAssay Description:All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a...More data for this Ligand-Target Pair
Affinity DataIC50: 7.5nMAssay Description:Further provided herein are methods of inhibiting HDAC8 mediated deacetylation of p53. In one aspect, the method includes contacting HDAC8 with a HDA...More data for this Ligand-Target Pair
Affinity DataIC50: >20nMAssay Description:Further provided herein are methods of inhibiting HDAC8 mediated deacetylation of p53. In one aspect, the method includes contacting HDAC8 with a HDA...More data for this Ligand-Target Pair
Affinity DataIC50: >20nMAssay Description:Further provided herein are methods of inhibiting HDAC8 mediated deacetylation of p53. In one aspect, the method includes contacting HDAC8 with a HDA...More data for this Ligand-Target Pair
Affinity DataIC50: >20nMAssay Description:Further provided herein are methods of inhibiting HDAC8 mediated deacetylation of p53. In one aspect, the method includes contacting HDAC8 with a HDA...More data for this Ligand-Target Pair
Affinity DataIC50: >20nMAssay Description:Further provided herein are methods of inhibiting HDAC8 mediated deacetylation of p53. In one aspect, the method includes contacting HDAC8 with a HDA...More data for this Ligand-Target Pair
Affinity DataIC50: >20nMAssay Description:Further provided herein are methods of inhibiting HDAC8 mediated deacetylation of p53. In one aspect, the method includes contacting HDAC8 with a HDA...More data for this Ligand-Target Pair
Affinity DataIC50: >20nMAssay Description:Further provided herein are methods of inhibiting HDAC8 mediated deacetylation of p53. In one aspect, the method includes contacting HDAC8 with a HDA...More data for this Ligand-Target Pair
Affinity DataIC50: 301nMAssay Description:All histone deacetylases were purchased from BPS Bioscience. The substrates, Broad Substrate A, and Broad Substrate B, were synthesized and are now a...More data for this Ligand-Target Pair