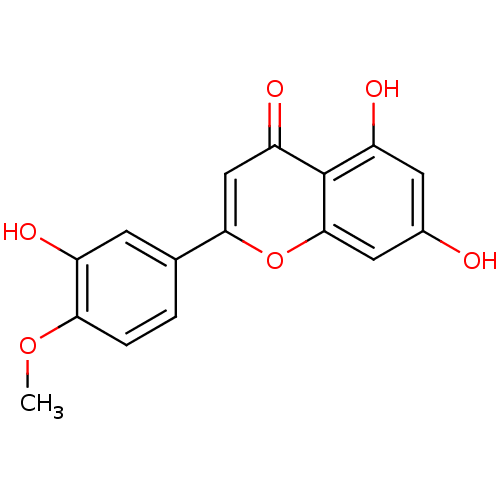
BDBM23414 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-chromen-4-one::Diosmetin::Diosmetin (25)
SMILES COc1ccc(cc1O)-c1cc(=O)c2c(O)cc(O)cc2o1
InChI Key InChIKey=MBNGWHIJMBWFHU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 23414
Found 15 hits for monomerid = 23414
Affinity DataKi: 16nMAssay Description:Inhibition of CYP1B1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufinMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Inhibition of CYP1B1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 89nMAssay Description:Inhibition of CYP1A1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufinMore data for this Ligand-Target Pair
Affinity DataIC50: 3.86E+3nMAssay Description:The kinase assay was performed using the EMD Millipore KinaseProfiler service assay protocol. Aurora B kinase was supplied by EMD Millipore Corp. The...More data for this Ligand-Target Pair
TargetMultidrug resistance-associated protein 1(Homo sapiens (Human))
The Hong Kong Polytechnic University
Curated by ChEMBL
The Hong Kong Polytechnic University
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of MRP1More data for this Ligand-Target Pair
TargetATP-binding cassette sub-family C member 2(Homo sapiens (Human))
The Hong Kong Polytechnic University
Curated by ChEMBL
The Hong Kong Polytechnic University
Curated by ChEMBL
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of MRP2More data for this Ligand-Target Pair
Affinity DataIC50: 2.44E+3nMAssay Description:Inhibition of human CYP1A2 by EROD assayMore data for this Ligand-Target Pair
Affinity DataIC50: 140nMAssay Description:Inhibition of human CYP1A1 by EROD assayMore data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Inhibition of human CYP1B1 by EROD assayMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 2.79E+4nMAssay Description:Inhibition of LSD1 (unknown origin) by fluorescence assayMore data for this Ligand-Target Pair
TargetInositol polyphosphate multikinase(Homo sapiens)
National Institute Of Environmental Health Sciences
Curated by ChEMBL
National Institute Of Environmental Health Sciences
Curated by ChEMBL
Affinity DataIC50: 7.20E+3nMAssay Description:Inhibition of human IPMK using insP3 as substrate preincubated for 15 mins followed by substrate and measured after 30 mins by TR-FRET assayMore data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 10(Homo sapiens (Human))
Eberhard Karls University Of Tuebingen
Eberhard Karls University Of Tuebingen
Affinity DataIC50: 2.86E+4nMT: 2°CAssay Description:The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n...More data for this Ligand-Target Pair
TargetInositol hexakisphosphate kinase 2(Homo sapiens)
National Institute Of Environmental Health Sciences
Curated by ChEMBL
National Institute Of Environmental Health Sciences
Curated by ChEMBL
Affinity DataIC50: 900nMAssay Description:Inhibition of human IP6K2 using insP6 as substrate preincubated for 15 mins followed by substrate and measured after 30 mins by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DatapH: 6.0 T: 2°CAssay Description:The assay was carried out at room temperature for 10 min with salivary alpha-amylase, starch, and test compounds. The reducing sugar was determined b...More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 14(Homo sapiens (Human))
Eberhard Karls University Of Tuebingen
Eberhard Karls University Of Tuebingen
Affinity DataIC50: 2.32E+4nMT: 2°CAssay Description:The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)