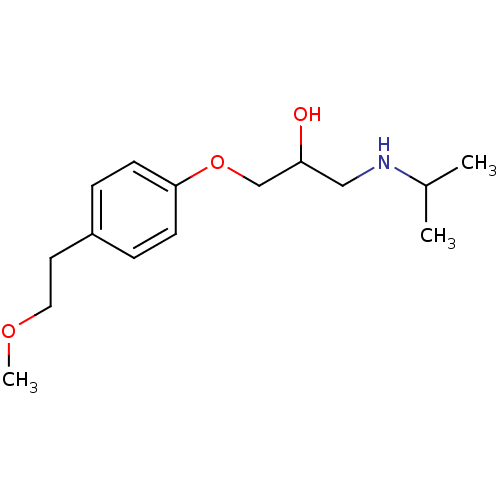
BDBM25756 (2R,3R)-2,3-dihydroxysuccinic acid;1-(isopropylamino)-3-[4-(2-methoxyethyl)phenoxy]propan-2-ol::Betalok::CHEMBL13::Metoprolol::Spesicor::Spesikor::metoprolol tartrate::{2-hydroxy-3-[4-(2-methoxyethyl)phenoxy]propyl}(propan-2-yl)amine
SMILES COCCc1ccc(OCC(O)CNC(C)C)cc1
InChI Key InChIKey=IUBSYMUCCVWXPE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 43 hits for monomerid = 25756
Found 43 hits for monomerid = 25756
TargetBeta-1 adrenergic receptor(Homo sapiens (Human))
UniversitÄT WÜRzburg
Curated by PDSP Ki Database
UniversitÄT WÜRzburg
Curated by PDSP Ki Database
TargetBeta-1 adrenergic receptor(Mus musculus)
Friedrich-Alexander Universit£T Erlangen-N£Rnberg
Curated by ChEMBL
Friedrich-Alexander Universit£T Erlangen-N£Rnberg
Curated by ChEMBL
Affinity DataKi: 49nMAssay Description:Displacement of [3H]CGP12177 from mouse beta1 adrenoceptor expressed in HEK293T cell membranesMore data for this Ligand-Target Pair
TargetBeta-1 adrenergic receptor(Homo sapiens (Human))
UniversitÄT WÜRzburg
Curated by PDSP Ki Database
UniversitÄT WÜRzburg
Curated by PDSP Ki Database
Affinity DataKi: 55nMAssay Description:Displacement of [3H]CGP12177 from human beta1 adrenoceptor expressed in HEK293T cell membranesMore data for this Ligand-Target Pair
TargetBeta-1 adrenergic receptor(Rattus norvegicus (Rat))
Niigata College Of Pharmacy
Curated by PDSP Ki Database
Niigata College Of Pharmacy
Curated by PDSP Ki Database
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Friedrich-Alexander Universit£T Erlangen-N£Rnberg
Curated by ChEMBL
Friedrich-Alexander Universit£T Erlangen-N£Rnberg
Curated by ChEMBL
Affinity DataKi: 220nMAssay Description:Displacement of [3H]CGP12177 from human beta2 adrenoceptor expressed in CHO cell membranesMore data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Rattus norvegicus)
Niigata College Of Pharmacy
Curated by PDSP Ki Database
Niigata College Of Pharmacy
Curated by PDSP Ki Database
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Friedrich-Alexander Universit£T Erlangen-N£Rnberg
Curated by ChEMBL
Friedrich-Alexander Universit£T Erlangen-N£Rnberg
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:Binding affinity of a compound to rat brain 5-hydroxytryptamine 1A (serotonin) receptor assayed by radiolabeled [3H]-8-OH-DPAT ligand displacementMore data for this Ligand-Target Pair
TargetBeta-3 adrenergic receptor(Homo sapiens (Human))
UniversitÄT WÜRzburg
Curated by PDSP Ki Database
UniversitÄT WÜRzburg
Curated by PDSP Ki Database
Target5-hydroxytryptamine receptor 1B(Rattus norvegicus (Rat))
Niigata College Of Pharmacy
Curated by PDSP Ki Database
Niigata College Of Pharmacy
Curated by PDSP Ki Database
TargetATP-dependent translocase ABCB1(Homo sapiens (Human))
Bulgarian Academy Of Sciences
Curated by ChEMBL
Bulgarian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 2.00E+5nMAssay Description:High affinity constant at binding site of human P-Glycoprotein (P-gp) in two-affinity modelMore data for this Ligand-Target Pair
Affinity DataKi: 2.30E+5nMAssay Description:Competitive inhibition of Capra hircus (goat) brain DPP-3 using Arg-Arg-4mbetaNA as substrate assessed as liberation of 4mbetaNA from substrate prein...More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2C9 in human liver microsomes using tolbutamide substrate by LC-MS/MS methodMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP1A2 in human liver microsomes using phenacetin substrate by LC-MS/MS methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.87E+3nMAssay Description:Inhibition of human recombinant CYP2J2 assessed as reduction in astemizole O-demethylation by LC-MS/MS methodMore data for this Ligand-Target Pair
TargetATP-dependent translocase ABCB1(Homo sapiens (Human))
Bulgarian Academy Of Sciences
Curated by ChEMBL
Bulgarian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.30E+6nMAssay Description:Concentration required for 50% inhibition at binding site of human P-Glycoprotein (P-gp) in one-affinity modelMore data for this Ligand-Target Pair
Affinity DataIC50: 1.17E+5nMAssay Description:Inhibition of binding of Batrachotoxinin [3H]BTX-B to high affinity sites on voltage dependent sodium channels in a vesicular preparation from guinea...More data for this Ligand-Target Pair
Affinity DataKd: 162nMAssay Description:In vitro beta-2 adrenergic receptor activity was determined by measuring inhibition of the isoproterenol induced relaxation in isolated guinea pig tr...More data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Tested for beta2-adrenergic blocking effect by measuring the ability to inhibit the relaxing effect of epinephrine on the isolated tracheal muscle of...More data for this Ligand-Target Pair
Affinity DataIC50: 320nMAssay Description:Tested for beta2-adrenergic blocking effect by measuring the ability to inhibit the relaxing effect of epinephrine on the isolated tracheal muscle of...More data for this Ligand-Target Pair
Affinity DataIC50: >1.35E+5nMAssay Description:Binding affinity towards peroxisome proliferator activated receptor alpha (PPAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: >1.33E+5nMAssay Description:Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: >1.33E+5nMAssay Description:Inhibition of human MRP2 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: >1.33E+5nMAssay Description:Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ...More data for this Ligand-Target Pair
Affinity DataIC50: >1.33E+5nMAssay Description:Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.15E+5nMAssay Description:Inhibition of Capra hircus (goat) brain DPP-3 using Arg-Arg-4mbetaNA as substrate assessed as liberation of 4mbetaNA from substrateMore data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Friedrich-Alexander Universit£T Erlangen-N£Rnberg
Curated by ChEMBL
Friedrich-Alexander Universit£T Erlangen-N£Rnberg
Curated by ChEMBL
Affinity DataKd: 1.55E+4nMAssay Description:Displacement of [3H]DHA from inactive/G protein-uncoupled human beta2-AR expressed in CHO cell membranes assessed as intrinsic Kd by liquid scintilla...More data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Friedrich-Alexander Universit£T Erlangen-N£Rnberg
Curated by ChEMBL
Friedrich-Alexander Universit£T Erlangen-N£Rnberg
Curated by ChEMBL
Affinity DataKd: 1.55E+4nMAssay Description:Displacement of [3H]DHA from inactive/G protein-uncoupled human beta2-AR expressed in CHO cell membranes assessed as intrinsic Kd by liquid scintilla...More data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Friedrich-Alexander Universit£T Erlangen-N£Rnberg
Curated by ChEMBL
Friedrich-Alexander Universit£T Erlangen-N£Rnberg
Curated by ChEMBL
Affinity DataKd: 130nMAssay Description:Displacement of [3H]DHA from inactive/G protein-uncoupled human beta2-AR expressed in CHO cell membranes by liquid scintillation countingMore data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Friedrich-Alexander Universit£T Erlangen-N£Rnberg
Curated by ChEMBL
Friedrich-Alexander Universit£T Erlangen-N£Rnberg
Curated by ChEMBL
Affinity DataKd: 130nMAssay Description:Displacement of [3H]DHA from inactive/G protein-uncoupled human beta2-AR expressed in CHO cell membranes by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using bufuralol substrate by LC-MS/MS methodMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2C19 in human liver microsomes using omeprazole substrate by LC-MS/MS methodMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP3A4 in human liver microsomes using testosterone substrate by LC-MS/MS methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+4nMAssay Description:Inhibitory activity against recombinant human Cytochrome P450 2D6 (CYP2D6) after incubated for 45 minutesMore data for this Ligand-Target Pair
Affinity DataKd: 15nMAssay Description:In vitro beta-1 adrenergic receptor activity was determined via inhibition of the positive chronotropic actions of isoproterenol in isolated guinea p...More data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Tested for beta1-adrenergic blocking effect by measuring the ability to inhibit the positive inotropic effect of isoproterenol on the isolated right ...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 2(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataEC50: 3.54E+4nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataEC50: 9.61E+3nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataEC50: 3.48E+4nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS...More data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 2(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataEC50: 1.62E+4nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS...More data for this Ligand-Target Pair
TargetBeta-3 adrenergic receptor(Homo sapiens (Human))
UniversitÄT WÜRzburg
Curated by PDSP Ki Database
UniversitÄT WÜRzburg
Curated by PDSP Ki Database
Affinity DataKd: 6.92E+3nMAssay Description:The whole cell-binding studies were undertaken in CHO cell lines stably expressing each beta-adrenoceptor subtype. Nonspecific binding was determined...More data for this Ligand-Target Pair
TargetBeta-1 adrenergic receptor(Homo sapiens (Human))
UniversitÄT WÜRzburg
Curated by PDSP Ki Database
UniversitÄT WÜRzburg
Curated by PDSP Ki Database
Affinity DataKd: 55nMpH: 7.4 T: 2°CAssay Description:The whole cell-binding studies were undertaken in CHO cell lines stably expressing each beta-adrenoceptor subtype. Nonspecific binding was determined...More data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Friedrich-Alexander Universit£T Erlangen-N£Rnberg
Curated by ChEMBL
Friedrich-Alexander Universit£T Erlangen-N£Rnberg
Curated by ChEMBL
Affinity DataKd: 129nMAssay Description:The whole cell-binding studies were undertaken in CHO cell lines stably expressing each beta-adrenoceptor subtype. Nonspecific binding was determined...More data for this Ligand-Target Pair