BDBM31773 ECONAZOLE::Econazole nitrate::Gyno-pevaryl::Pevaryl::cid_68589
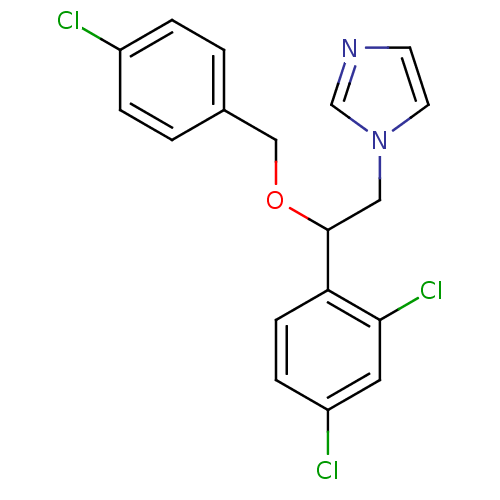
SMILES Clc1ccc(COC(Cn2ccnc2)c2ccc(Cl)cc2Cl)cc1
InChI Key InChIKey=LEZWWPYKPKIXLL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 32 hits for monomerid = 31773
Found 32 hits for monomerid = 31773
Affinity DataKi: 325nMAssay Description:In vitro inhibition of human Cytochrome P450 17A1 activityMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily M member 2(Homo sapiens (Human))
Peking University
Curated by ChEMBL
Peking University
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human TRPM2 expressed in HEK293T cells assessed as blocked of ADPR-activated current by whole cell patch clamp electrophysiologyMore data for this Ligand-Target Pair
TargetNeuropeptide Y receptor type 1(Homo sapiens (Human))
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 5.15E+3nMAssay Description:Source (MLSCN Center Name): The Scripps Research Institute Molecular Screening Center Affiliation: The Scripps Research Institute, TSRI Assay Provide...More data for this Ligand-Target Pair
Affinity DataEC50: 1.75E+4nMAssay Description:Keywords: GFP, refolding, reducing reagent, GFP fluorescence Assay Overview: M. tuberculosis harbors three inteins, which interrupt the DnaB, RecA, a...More data for this Ligand-Target Pair
Affinity DataEC50: 1.02E+4nMAssay Description:Keywords: GFP-RecA intein, protein splicing, refolding, reducing reagent, GFP fluorescence Assay Overview: M. tuberculosis harbors three inteins, whi...More data for this Ligand-Target Pair
TargetUbiquitin-conjugating enzyme E2 N(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: >2.00E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego CA...More data for this Ligand-Target Pair
Affinity DataKd: 780nMAssay Description:Substrate and ligand binding assay using UV- visible absorbance analysis of CYP142 was done on a Cary UV-50 UV-visible scanning spectrophotometer (Va...More data for this Ligand-Target Pair
Affinity DataKd: 1.17E+4nMAssay Description:Substrate and ligand binding assay using UV- visible absorbance analysis of CYP142 was done on a Cary UV-50 UV-visible scanning spectrophotometer (Va...More data for this Ligand-Target Pair
Affinity DataKd: 4.60E+3nMAssay Description:Substrate and ligand binding assay using UV- visible absorbance analysis of CYP142 was done on a Cary UV-50 UV-visible scanning spectrophotometer (Va...More data for this Ligand-Target Pair
Affinity DataKd: 1.93E+3nMAssay Description:Binding assay using CYP130, CYP130 (G234) or CYP142.More data for this Ligand-Target Pair
Affinity DataKd: 4.50E+3nMAssay Description:Binding assay using CYP130, CYP130 (G234) or CYP142.More data for this Ligand-Target Pair
Affinity DataKd: 330nMAssay Description:Binding assay using CYP130, CYP130 (G234) or CYP142.More data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Homo sapiens (Human))
Swiss Institute Of Bioinformatics
Curated by ChEMBL
Swiss Institute Of Bioinformatics
Curated by ChEMBL
Affinity DataIC50: 8.10E+3nMAssay Description:Inhibition of IDO1 (unknown origin) using L-tryptophan substrate incubated for 60 mins by HPLCMore data for this Ligand-Target Pair
Affinity DataKd: 73nMAssay Description:Binding affinity to Mycobacterium tuberculosis H37Rv wild type CYP121 by titration assayMore data for this Ligand-Target Pair
TargetLanosterol 14-alpha demethylase(Mycobacterium tuberculosis (strain CDC 1551 / Oshk...)
Swansea University
Curated by ChEMBL
Swansea University
Curated by ChEMBL
Affinity DataKd: 200nMAssay Description:Binding affinity to Mycobacterium tuberculosis CYP51More data for this Ligand-Target Pair
TargetMalate dehydrogenase, cytoplasmic(Homo sapiens (Human))
Northwestern University
Curated by ChEMBL
Northwestern University
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of malate dehydrogenase (MDH)More data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+5nMAssay Description:Compound was tested for the inhibition of ChymotrypsinogenMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Compound was tested for the inhibition of beta-lactamaseMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of human CYP51 expressed in Topp 3 cells by lanosterol demethylase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.60E+3nMAssay Description:Inhibition of mouse Ido2 transfected in HEK293T cells using L-tryptophan as substrate assessed as kynurenine formation after 45 mins by spectrophotom...More data for this Ligand-Target Pair
Affinity DataIC50: 1.12E+4nMAssay Description:Inhibition of mouse Ido1 transfected in HEK293T cells using L-tryptophan as substrate assessed as kynurenine formation after 45 mins by spectrophotom...More data for this Ligand-Target Pair
TargetCalcium release-activated calcium channel protein 1(Homo sapiens (Human))
The University Of Queensland
Curated by ChEMBL
The University Of Queensland
Curated by ChEMBL
Affinity DataIC50: 1.70E+4nMAssay Description:Inhibition of Orai1-mediated store operated Ca2+ entry in human MDA-MB-231 cells assessed as reduction in BAPTA-induced Ca2+ depletion-stimulated SOC...More data for this Ligand-Target Pair
TargetCalcium release-activated calcium channel protein 1(Homo sapiens (Human))
The University Of Queensland
Curated by ChEMBL
The University Of Queensland
Curated by ChEMBL
Affinity DataIC50: 9.40E+4nMAssay Description:Inhibition of Orai1-mediated store operated Ca2+ entry in human MDA-MB-231 cells assessed as reduction of SERCA inhibition-induced ER release preincu...More data for this Ligand-Target Pair
Affinity DataIC50: 1.34E+4nMAssay Description:Inhibition of recombinant human BSEP expressed in baculovirus infected sf9 cell membrane vesicles assessed as reduction in ATP or AMP-dependent [3H]-...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+4nMAssay Description:Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-...More data for this Ligand-Target Pair
Affinity DataIC50: 1.74E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
China Pharmaceutical University
Curated by ChEMBL
China Pharmaceutical University
Curated by ChEMBL
Affinity DataIC50: 2.23E+4nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 2 m...More data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Homo sapiens (Human))
Swiss Institute Of Bioinformatics
Curated by ChEMBL
Swiss Institute Of Bioinformatics
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of human recombinant IDO1 using L-tryptophan as substrate after 30 mins by fluorimetric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 430nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+4nMpH: 7.4 T: 2°CAssay Description:HO activity in rat spleen (HO-1) and brain (HO-2) microsomal fractions was determined by the quantitation of CO formed from the degradation of methem...More data for this Ligand-Target Pair
Affinity DataIC50: 4.90E+4nMpH: 7.4 T: 2°CAssay Description:HO activity in rat spleen (HO-1) and brain (HO-2) microsomal fractions was determined by the quantitation of CO formed from the degradation of methem...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)