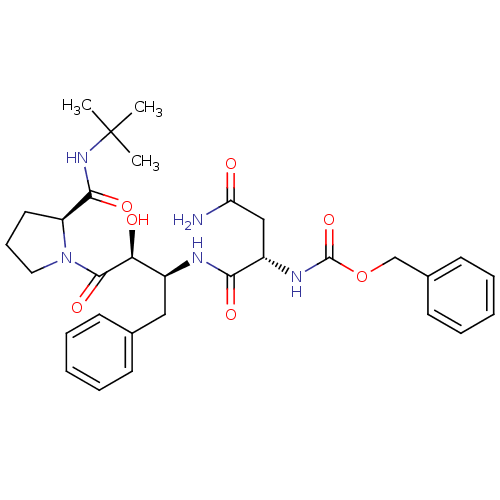
BDBM4215 AHPBA 1a::benzyl N-[(1S)-1-{[(2S,3S)-4-[(2S)-2-(tert-butylcarbamoyl)pyrrolidin-1-yl]-3-hydroxy-4-oxo-1-phenylbutan-2-yl]carbamoyl}-2-carbamoylethyl]carbamate
SMILES CC(C)(C)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)OCc1ccccc1
InChI Key InChIKey=XCVUOCMQYKSJJR-IGRGDXOOSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 4215
Found 4 hits for monomerid = 4215
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Syntex Research
Curated by ChEMBL
Syntex Research
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Binding affinity against HIV Protease enzyme.(by Dixon analysis)More data for this Ligand-Target Pair
Affinity DataKi: 57.5nM ΔG°: -10.3kcal/molepH: 4.7 T: 2°CAssay Description:The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as...More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Syntex Research
Curated by ChEMBL
Syntex Research
Curated by ChEMBL
Affinity DataIC50: 7.40nMAssay Description:Inhibitory activity of the Compound was tested against HIV protease enzyme.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Syntex Research
Curated by ChEMBL
Syntex Research
Curated by ChEMBL
Affinity DataIC50: 7.40nMAssay Description:Inhibitory potency against HIV-1 proteaseMore data for this Ligand-Target Pair