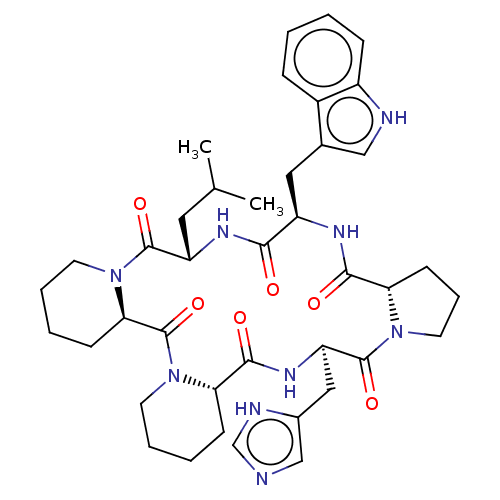
BDBM50001328 24-(1H-4-imidazolylmethyl)-16-(1H-3-indolylmethyl)-13-isobutyl-(6aR,16R,18aS,24R,26aS)-perhydrodipyrido[1,2-a:1,2-d]pyrrolo[1,2-j][1,4,7,10,13,16]hexaazacyclooctadecine-6,12,15,18,23,26-hexaone::CHEMBL338020
SMILES CC(C)C[C@@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCCN2C(=O)[C@H]2CCCCN2C1=O
InChI Key InChIKey=LCKIMAYTFGNSOJ-SRMNXDGMSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50001328
Found 3 hits for monomerid = 50001328
Affinity DataIC50: 3.00E+3nMAssay Description:Concentration required to displace 50% of [3H]arginine vasopressin from rat liver arginine vasopressin 1A (AVP-V1a) site.More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of [3H]arginine vasopressin binding to AVP-V2 site in rat kidney medulla.More data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Concentration required to displace 50% of [3H]oxytocin from rat uterine receptor.More data for this Ligand-Target Pair