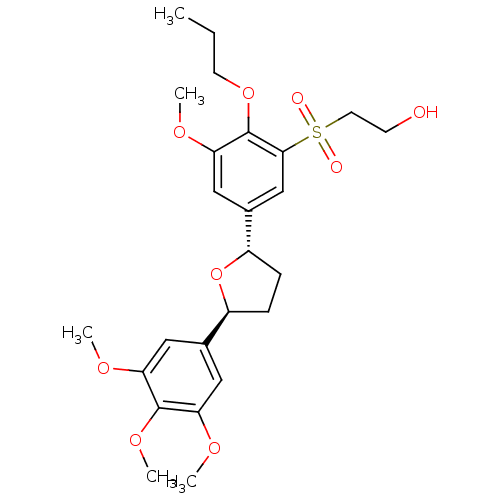
BDBM50002823 2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimethoxy-phenyl)-tetrahydro-furan-2-yl]-benzenesulfonyl}-ethanol::CHEMBL299944::L-680573::MK-287
SMILES CCCOc1c(OC)cc(cc1S(=O)(=O)CCO)[C@@H]1CC[C@H](O1)c1cc(OC)c(OC)c(OC)c1
InChI Key InChIKey=WXIDMVGKJBAEFP-OALUTQOASA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50002823
Found 9 hits for monomerid = 50002823
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:In vitro effect on inhibition of the binding of [3H]C18-Platelet activating factor to human PMN membranes preparationMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:In vitro inhibition of [3H]-C18 PAF binding to human platelet membrane Platelet activating factor receptor was determinedMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 6.10nMAssay Description:Compound was tested for it''s ability to inhibit [3H]C18-PAF binding to PMN membrane receptorsMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 6.30nMAssay Description:Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparationMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Antagonist activity against platelet activating factor (PAF) receptor in rabbit platelet membranes using [3H]C18-PAF as radioligandMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 59nMAssay Description:Compound was tested for it''s ability to inhibit [3H]C18-PAF binding to human plateletMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 116nMAssay Description:Compound was tested for it''s ability to inhibit [3H]C18-PAF binding to human plateletMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 116nMAssay Description:Compound was tested for it''s ability to inhibit [3H]C18-PAF binding to human plateletMore data for this Ligand-Target Pair
TargetPlatelet-activating factor receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Displacement of [3H]-PAF from PAF receptor of human platelet membranesMore data for this Ligand-Target Pair