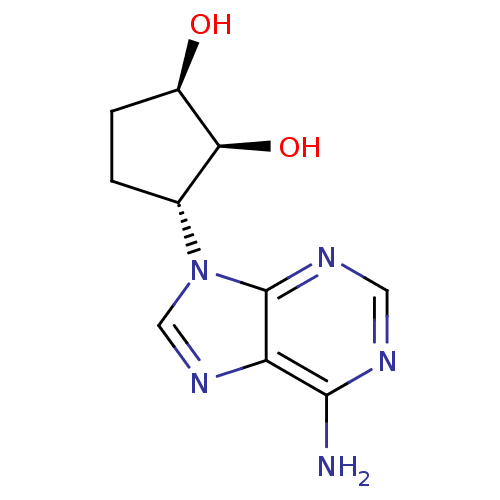
BDBM50006218 (1R,2S,3R)-3-(6-amino-9H-purin-9-yl)cyclopentane-1,2-diol::3-(6-Amino-purin-9-yl)-cyclopentane-1,2-diol::CHEMBL301499
SMILES Nc1ncnc2n(cnc12)[C@@H]1CC[C@@H](O)[C@H]1O
InChI Key InChIKey=CZASVOKHBMDKGF-JKMUOGBPSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50006218
Found 4 hits for monomerid = 50006218
Affinity DataKi: 12nMAssay Description:Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy).More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+4nMAssay Description:Inhibition of human recombinant SAHH at 1000 uMMore data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 minsMore data for this Ligand-Target Pair