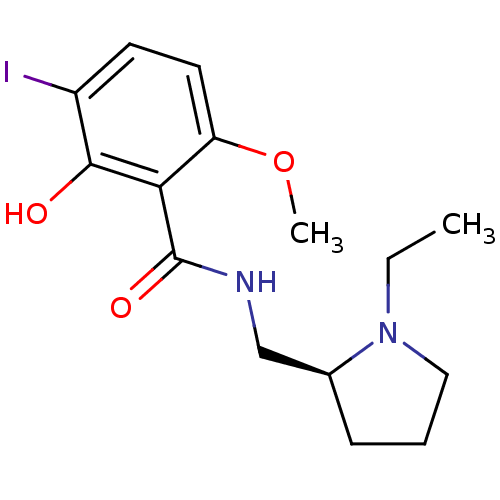
BDBM50012961 (-)-(S)-N-((1-ethylpyrrolidin-2-yl)methyl)-2-hydroxy-3-iodo-6-methoxybenzamide::(S)-N-((1-ethylpyrrolidin-2-yl)methyl)-2-hydroxy-3-iodo-6-methoxybenzamide::CHEMBL267723::N-((S)-1-Ethyl-pyrrolidin-2-ylmethyl)-2-hydroxy-3-iodo-6-methoxy-benzamide::N-(1-Ethyl-pyrrolidin-2-ylmethyl)-2-hydroxy-3-iodo-6-methoxy-benzamide::N-(1-Ethyl-pyrrolidin-2-ylmethyl)-2-hydroxy-3-iodo-6-methoxy-benzamide (IBZM)::N-(1-Ethyl-pyrrolidin-2-ylmethyl)-2-hydroxy-3-iodo-6-methoxy-benzamide( (S)-(-)IBZM)
SMILES CCN1CCC[C@H]1CNC(=O)c1c(O)c(I)ccc1OC
InChI Key InChIKey=CANPFCFJURGKAX-JTQLQIEISA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 13 hits for monomerid = 50012961
Found 13 hits for monomerid = 50012961
Affinity DataKi: 0.261nMAssay Description:In vitro inhibition of rat liver dihydrofolate reductase.More data for this Ligand-Target Pair
Affinity DataKi: 0.430nMAssay Description:Inhibition constant against dopamine receptor D2 in ratMore data for this Ligand-Target Pair
Affinity DataKi: 0.633nMAssay Description:Inhibitory constant against binding of [125I]- IBZM to rat striatal membraneMore data for this Ligand-Target Pair
Affinity DataKi: 4.20nMAssay Description:Displacement of [3H]spiperone from dopamine D2short receptor in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 4.20nMAssay Description:Displacement of [3H]spiperone from dopamine D3 receptor in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 4.20nMAssay Description:Displacement of [3H]spiperone from dopamine D2long receptor in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nMAssay Description:Displacement of [3H]SCH23990 from dopamine D1 receptor in pig stratial membraneMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:In vivo inhibitory activity of the compound against dopamine (D2) receptor in rat caudate-putamen tissueMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40nMAssay Description:In vitro binding affinity at Dopamine receptor D2 in rat by displacing [3H]- spiperone from rat striatal membraneMore data for this Ligand-Target Pair
Affinity DataKd: 0.430nMAssay Description:Evaluated in vivo for the affinity towards Dopamine receptor D2 of rat striatal tissueMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:In vivo binding affinity of the compound against dopamine (D1) receptor in rat caudate-putamen tissue using [3H]-SCH-23,390 as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:In vivo binding affinity of the compound against dopamine (D2) receptor in rat caudate-putamen tissue using [3H]-nemonapride as radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:In vivo inhibitory activity of the compound against dopamine (D1) receptor in rat caudate-putamen tissueMore data for this Ligand-Target Pair