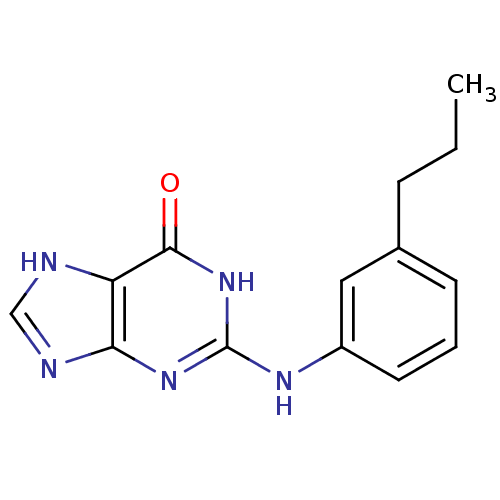
BDBM50013085 2-(3-Propyl-phenylamino)-1,9-dihydro-purin-6-one::CHEMBL63910
SMILES CCCc1cccc(Nc2nc3nc[nH]c3c(=O)[nH]2)c1
InChI Key InChIKey=UJLDHQAWSWICJD-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50013085
Found 4 hits for monomerid = 50013085
TargetThymidine kinase(Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...)
University Of Massachusetts Medical School
Curated by ChEMBL
University Of Massachusetts Medical School
Curated by ChEMBL
Affinity DataIC50: 3.31E+3nMAssay Description:Inhibitory activity against HSV-1 thymidine kinase isolated from HeLa cellsMore data for this Ligand-Target Pair
TargetThymidine kinase(Human herpesvirus 1 (strain SC16) (HHV-1) (Human h...)
University Of Massachusetts Medical School
Curated by ChEMBL
University Of Massachusetts Medical School
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of TdR phosphorylation using thymidine kinase (TK) assay for herpes simplex virus type 2 (HSV-2).More data for this Ligand-Target Pair
TargetThymidine kinase(Human herpesvirus 1)
University Of Massachusetts Medical School
Curated by ChEMBL
University Of Massachusetts Medical School
Curated by ChEMBL
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of TdR phosphorylation using thymidine kinase assay for herpes simplex virus type 1More data for this Ligand-Target Pair
TargetThymidine kinase(Human herpesvirus 2)
University Of Massachusetts Medical School
Curated by ChEMBL
University Of Massachusetts Medical School
Curated by ChEMBL
Affinity DataIC50: 3.02E+3nMAssay Description:Inhibitory activity against HSV-2 thymidine kinase isolated from HeLa cellsMore data for this Ligand-Target Pair