BDBM50013129 CHEMBL3261984
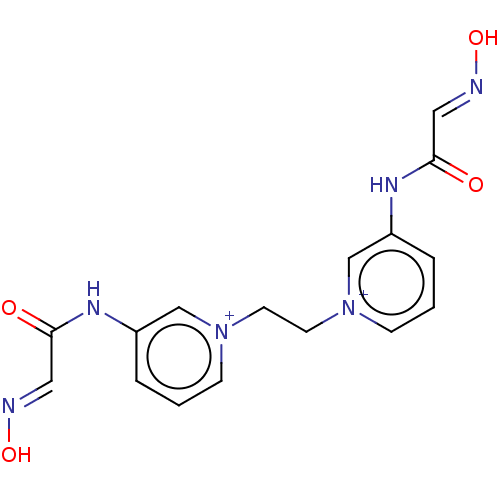
SMILES [Br-].[Br-].O\N=C\C(=O)Nc1ccc[n+](CC[n+]2cccc(NC(=O)\C=N\O)c2)c1
InChI Key InChIKey=FVWIMBQJMPHLTM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50013129
Found 3 hits for monomerid = 50013129
TargetAcetylcholinesterase(Homo sapiens (Human))
Defence Research & Development Establishment (Drde)
Curated by ChEMBL
Defence Research & Development Establishment (Drde)
Curated by ChEMBL
Affinity DataKd: 4.87E+4nMAssay Description:Binding affinity to VX-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured up ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Defence Research & Development Establishment (Drde)
Curated by ChEMBL
Defence Research & Development Establishment (Drde)
Curated by ChEMBL
Affinity DataKd: 4.29E+4nMAssay Description:Binding affinity to sarin-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured ...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Defence Research & Development Establishment (Drde)
Curated by ChEMBL
Defence Research & Development Establishment (Drde)
Curated by ChEMBL
Affinity DataIC50: 2.53E+6nMAssay Description:Inhibition of hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured up to 1 hr by Ellman m...More data for this Ligand-Target Pair